Background and overview[1]
Methylene isC10 A highly valuable component among heavy aromatics, it is mainly used to produce pyromellitic dianhydride (PMDA). The main use of pyromellitic dianhydride is to react with 4,4-diaminodiether to produce new high-temperature resistant engineering plastics and insulating materials polyimide (PI). Polyimide is widely used in aerospace, atomic energy, electromechanical and other industrial production due to its excellent electrical insulation properties, high temperature resistance and radiation resistance. In addition, PMDA is also an important raw material for high-quality plasticizers, curing agents and matting agents for powder coatings. The demand is increasing, and the demand for pyrene has also doubled.
Preparation[1]
The production methods of pyrene can be divided into synthesis method and separation method. Synthetic methods have been gradually phased out due to high production costs. At present, pyrene at home and abroad is mainly obtained from C10 heavy aromatics by separation methods. C10 heavy aromatics mainly come from by-products in the production process such as catalytic reforming in refineries and wide-cut reforming in chemical fiber plants. Most of them are burned as fuel oil or to produce low-grade aromatic solvents, causing great waste of resources and environmental pollution. pollute. It is of great practical significance to comprehensively utilize C10 aromatic hydrocarbon resources and develop its downstream high value-added fine chemical products. The production method of pyrene in our country is in the development stage. The small-scale experiments on the gas-phase isomerization of tetramethylbenzene and the liquid-phase isomerization of tetramethylbenzene have been completed and are in the factory-fit stage. At present, the production of pyrene in my country has been industrialized. The main manufacturers are Yangzhou Hualun Chemical Company, Jiangsu Liyang Solvent Factory, Jinling Petrochemical Company Refinery, Taizhou Chemical Plant, Heilongjiang Anda Chemical Plant, Nanjing Changlu Xufeng Chemical Factory, Jiangsu Changshu Federal Chemical Factory, etc.
1 Separation method of C10 heavy aromatics
The output of paraxylene (PX), the raw material for the production of polyester, has increased significantly in recent years, and the PX unit has heavy aromatic by-products. In general designs, heavy aromatics are sent directly to gasoline and diesel storage tanks for blending. However, due to the low hydrogen-to-carbon ratio and wide distillation range of heavy aromatics, they affect the dry point of gasoline, reduce the cetane number of diesel and contain condensed rings in the fuel exhaust. Due to aromatic hydrocarbons and other reasons, it is not suitable for internal combustion engine fuel. A domestic refinery unit makes full use of the heavy aromatic hydrocarbons produced by the PX unit. Through distillation, freeze crystallization, centrifugal separation and other processes, it can separate the heavy aromatic hydrocarbons that are not easily utilized into 1# solvent oil, 2# solvent oil, 3# solvent oil and so on. #The three types of solvent oils produced by products such as solvent oil and high value-added pylene tetramethylbenzene are all products in short supply in the market, and their added value is higher than the value of raw materials. Methyl tetramethylbenzene is a high value-added fine chemical raw material. The heavy aromatics raw material used in the unit is a by-product of the aromatics complex unit of the refinery, and its composition is shown in Table 1. The physical and chemical indicators of the produced products are shown in Table 2.
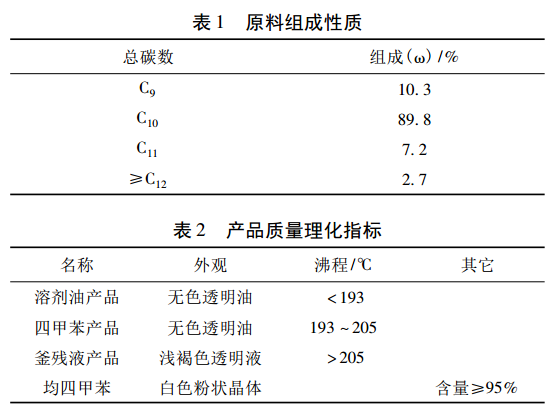
As shown in Figure 1, the process flow of this device includes two parts: heavy aromatic hydrocarbon fractionation and freezing separation. It adopts the process technology route of vacuum intermittent distillation heated by heat carrier, refrigeration crystallization with cold brine, and natural sweating. The auxiliary systems of the device include thermal oil furnace system and cold brine refrigeration system.
With the continuous expansion of domestic PX production capacity, the separation of high value-added fine chemical products from the by-product heavy aromatics can not only solve the problem of the outlet of the by-product, but also create good economic benefits. The construction of a heavy aromatic hydrocarbons separation device requires low investment and quick results, and can flexibly adjust product solutions to meet market demand in the face of changing market conditions.
Isomerization of tetramethylbenzene in 2 C10 aromatics
Methylene (1,2,3,5-tetramethylbenzene) is produced by liquid phase isomerization under normal pressure and lower reaction temperature. Li Huimin et al. developed a two-component solid acid catalyst. In order to suppress the disproportionation reaction, a certain amount of trimethylbenzene was added to the reaction system to fully proceed with the main reaction and obtain higher yield of mesitylene. The experimental results are shown in Table 3.
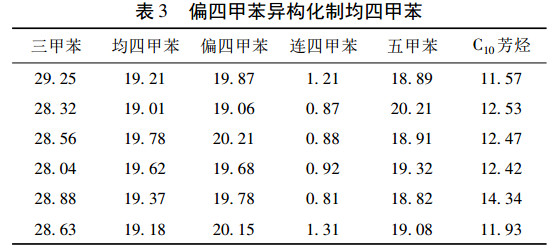
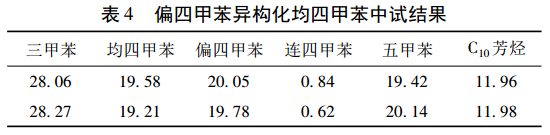
On the basis of the small-scale trial, a pilot plant with an annual output of 50 t was carried out. The isomerization reactor was a 1000 L enamel kettle, the one-time feeding volume was 300 kg, the reaction temperature was 120 ℃, the reaction time was 4 h, and 20% was added (ω) trimethylbenzene, the catalyst addition amount is 0. 9 kg. The experimental results are shown in Table 4.
At present, solid acid catalysts, especially zeolite catalysts, are most widely used in the industrial process of aromatic hydrocarbon isomerization. In the isomerization reaction, whether the raw materials and products can smoothly enter and leave the zeolite pores as required is the main basis for selecting the zeolite catalyst. Mordenite is a twelve-membered ring with a large pore size. Its cross-section is oval and the average pore diameter is 0. 66 nm. It can adsorb molecules such as benzene and cycloalkanes. Mordenite also has good acid resistance, heat resistance and catalytic activity, and can be used as a catalyst for the isomerization of tetramethylbenzene.
The technology of isomerizing tetramethylbenzene to pyrene under the action of mordenite catalyst has been studied for a long time, and the reaction process needs to be carried out under hydrogen conditions. Theoretically, hydrogen is not required for the isomerization of tetramethylbenzene. The presence of hydrogen is beneficial to inhibit the formation of coke on the catalyst and extend the life of the catalyst. However, the hydrogen reaction process is relatively complex, has poor operational safety, and is limited by the source of hydrogen. It can be seen from the results of small and pilot experiments that the isomerization reaction of tetramethylbenzene is used to prepare pyrene. The content of pyrene in the product is stable and is easy to be scaled up industrially.
3 Trimethylbenzene methanol alkylation
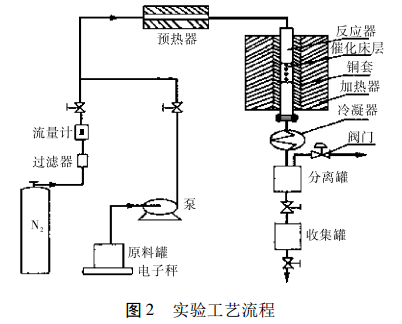
Experiments generally use a tubular isothermal reactor with a copper sleeve on the outside of the tube. The catalyst is evenly mixed with the same porcelain ring at a ratio of 1:1 (V/V). The upper and lower parts of the catalyst bed are filled with porcelain rings. Trimethylbenzene and methanol are uniformly mixed in a certain ratio, then added to the raw material tank, and injected into the reactor by an advection pump. The process flow is shown in Figure 2.
The boiling points of tetramethylbenzene and tetramethylbenzene are very similar (the difference is 2. 2°C), and the content of tetramethylbenzene must be strictly controlled. Through the product analysis results, the conversion rate x of mesitylene, the yield y of mesitylene, the selectivity s of mesitylene, and the ratio c of mesitylene in tetramethylbenzene can be calculated. The activity stability period of the catalyst is short. In the early stage of the reaction, the conversion rate of mesitylene dropped from 45% to 20%. Subsequently, the reaction temperature needs to be continuously increased to maintain the activity of the catalyst. As the reaction time increases, the increase in conversion rate gradually becomes smaller after increasing the reaction temperature. The selectivity of pyrene gradually increased during the reaction. The proportion of mesitylene in tetramethylbenzene increased from 90% to 98% in the early stage of the reaction, was stable in the middle stage of the reaction, and decreased slightly in the later stage.
During the reaction time of 280 h, the reaction temperature increased from 350°C to 435°C. The average values of the main parameters were: the conversion rate of mesitylene was 24. 2%, and the selectivity of mesitylene was 85. 0%, the proportion of tetramethylbenzene is 96.8%. The deactivated catalyst in the above experiment was placed in a muffle furnace and baked at 550°C for 4 hours to regenerate the HZSM-5 catalyst. The regenerated catalyst returned from black to its original white color. The 280-h life span of the regenerated catalyst was investigated, and it was found that within the reaction time of 280 h, the regenerated catalyst basically reached the reaction performance of the fresh catalyst, indicating that carbon deposition was the main cause of catalyst deactivation.
Main reference materials
[1]Liang Jianyou. Production method of pylenetetramethylbenzene[J]. Guangzhou Chemical Industry, 2013, 41(20):148-149+188.
ice:100%; border-image-source:none; border-image- border-left-color:rgb(51, 51, 51); border-left-style:none; border-left- border-right-color: rgb(51, 51, 51); border-right-style:none; border-right- border-top-color:rgb(51, 51, 51); border-top-style:none; border-top- box- sizing:border-box; color:rgb(51, 51, 51); font-family:& font-size:13px; font-style:normal; font-variant:normal; font-weight:400; letter-spacing: normal; list-style-image:none; list-style-position:outside; list-style-type:none; margin-bottom:0px; margin-left:0px; margin-right:0px; margin-top:0px; orphans:2; padding-bottom:0px; padding-left:0px; padding-right:0px; padding-top:0px; text-align:left; text-decoration:none; text-indent:0px; text-transform: none; vertical-align:middle; white-space:normal; word-spacing:0px” />
The boiling points of tetramethylbenzene and tetramethylbenzene are very similar (the difference is 2. 2°C), and the content of tetramethylbenzene must be strictly controlled. Through the product analysis results, the conversion rate x of mesitylene, the yield y of mesitylene, the selectivity s of mesitylene, and the ratio c of mesitylene in tetramethylbenzene can be calculated. The activity stability period of the catalyst is short. In the early stage of the reaction, the conversion rate of mesitylene dropped from 45% to 20%. Subsequently, the reaction temperature needs to be continuously increased to maintain the activity of the catalyst. As the reaction time increases, the increase in conversion rate gradually becomes smaller after increasing the reaction temperature. The selectivity of pyrene gradually increased during the reaction. The proportion of mesitylene in tetramethylbenzene increased from 90% to 98% in the early stage of the reaction, was stable in the middle stage of the reaction, and decreased slightly in the later stage.
During the reaction time of 280 h, the reaction temperature increased from 350°C to 435°C. The average values of the main parameters were: the conversion rate of mesitylene was 24. 2%, and the selectivity of mesitylene was 85. 0%, the proportion of tetramethylbenzene is 96.8%. The deactivated catalyst in the above experiment was placed in a muffle furnace and baked at 550°C for 4 hours to regenerate the HZSM-5 catalyst. The regenerated catalyst returned from black to its original white color. The 280-h life span of the regenerated catalyst was investigated, and it was found that within the reaction time of 280 h, the regenerated catalyst basically reached the reaction performance of the fresh catalyst, indicating that carbon deposition was the main cause of catalyst deactivation.
Main reference materials
[1]Liang Jianyou. Production method of pylenetetramethylbenzene[J]. Guangzhou Chemical Industry, 2013, 41(20):148-149+188.

 微信扫一扫打赏
微信扫一扫打赏

