[Background and Overview][1][2][3]
Pyrogallic acid is a chemical reagent and chemical raw material with various uses. It is widely used in pharmaceutical synthesis, dyes, food, pesticides and electronic products, etc., as a developer, heat sensitive agent and auxiliary agent for polymer materials. reagents and chemical analysis reagents, etc., and in some fields cannot be replaced by other products. The main raw material for the current production of pyrogallic acid is gallic acid. Gallic acid is produced from the hydrolysis of the forest product gallnut or tara. Galla gallnut is a specialty product of my country, with good quality and large quantity (the output accounts for about 1/4 of the world’s output). Therefore, deep processing of gallnuts has great economic and social benefits.
Pyrogallic acid is an important organic fine chemical product, widely used in chemical industry, organic synthesis, printing and dyeing, analysis, food, medicine, pesticides and other fields. Mainly used as oxygen absorber, photographic developer and heat sensitive agent, radioactive element collector, dye mordant, metal salt reducing agent, neutral electroplating, chloroprene rubber vulcanization and PVC polymerization kettle additive, food packaging, synthetic resin Antioxidants. For example, the heart-brain infusion blood agar medium was used to study the inhibitory effect of propolis on Helicobacter pylori using the pyrogallic acid method, and ideal results were achieved. This method has the advantages of easy operation and low cost. Helicobacter pylori infection is a very common chronic bacterial infection and the main cause of chronic gastritis and peptic ulcer. This method is low in cost and has good curative effect, is in line with my country’s national conditions and people’s sentiments, and has great use value.
[Characteristics and Structure][4][5]
This product is a white shiny crystal with a melting point of 131~134°C, a boiling point of 309°C, a relative density of 1.46, and UVλmax2. 88nm in water at pH = 5.4. Easily soluble in water, alcohol and ether, slightly soluble in benzene, chloroform and carbon disulfide. Heating can sublimate. It will turn brown when exposed to light or in the air. When the solution is alkaline, it quickly absorbs oxygen and changes color. This product is toxic, with a toxicity LD50789mg/kg (oral administration to mice). It has extremely strong reducibility and can also undergo substitution reactions on the benzene ring.
[Quality Standard][4]

[Production process][2][4]
There are many methods for preparing pyrogallic acid, which are mainly divided into two categories: one is through chemical processing of forest products, and the gallic acid obtained from gallic acid tannin or tartannin is heated and decarboxylated to generate pyrogallic acid; The other type is to obtain pyrogallic acid through chemical synthesis
1. Through chemical processing of forest products
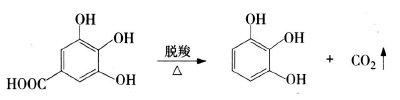
1) Preparation of pyrogallic acid using gallic acid as raw material: Gallic acid is decarboxylated and purified to obtain pyrogallic acid. Currently, the decarboxylation methods commonly used in industry include: biological decarboxylation, atmospheric pressure catalytic decarboxylation and reduced pressure catalytic decarboxylation. The reaction formula is as follows:
2) One-step preparation of pyrogallic acid using Tara as raw material: Using Tara powder as raw material to prepare pyrogallic acid, the product can reach reagent grade standards. Preparation process: Mix tara powder and water in a ratio of 1:3 (g:mL), and make lime paste with a mass of about 20% of tara powder and put it into a pressure-resistant reactor. Stir evenly and cover it. Seal it, put it in the oven, slowly raise the temperature to 200°C, and keep it warm for 3 hours. After the reaction is completed, cool down, add hydrochloric acid for decarboxylation, filter to remove the residue, then extract with ethyl acetate, and evaporate the ester layer to dryness. The residue is extracted with xylene to obtain a crude product, which is then decolorized and refined to obtain white needle-like crystals of pyrogallic acid. The one-step method greatly shortens the process flow, is easy to operate, reduces investment, has a short growth cycle, and uses cheaper raw materials, which can achieve greater economic benefits.
2. Preparation of pyrogallic acid by chemical synthesis
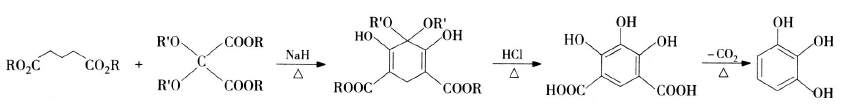
1) Hurd synthesis route: Hurd and Shipchandler chemically synthesized pyrogallic acid for the first time in the 1970s. This method uses fatty acid esters (such as glutarate) as raw materials, performs a condensation cyclization reaction in the presence of a catalyst, and the cyclization product is hydrolyzed under hydrogen chloride conditions and decarboxylated to generate pyrogallic acid. The reaction equation is as follows:
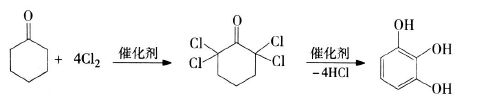
2) Synthesis of pyrogallic acid using cyclohexanone as raw material: 2,2,6,6-tetrachlorocyclohexanone is first produced by chlorination of cyclohexanone, and then catalytically hydrolyzed to produce pyrogallic acid. The process is simple and the raw materials come from a wide range of sources. Many scientific research institutions have conducted extensive research on this process. CCl4 or petroleum ether is usually used as the solvent, SOCl2 and SeOCl2 are used as the chlorination catalyst, and morpholine, citrate, etc. are used as the hydrolysis catalyst. The two-step yield can reach 79.7%. The reaction equation is as follows:
3) Synthesis of pyrogallic acid using cyclohexene as raw material: Japan has conducted systematic research on this process and obtained a number of patents. Using vanadium naphthenate as a catalyst, cyclohexene is oxidized with air, and then hydrolyzed to obtain 1,2,3-cyclohexanetriol, which is dehydrogenated under inert gas protection and catalytic conditions to generate pyrogallic acid. The reaction formula is as follows:
<p style="text-align: center;cause serious harm. The combination of citric acid, vitamin C and pyrogallic acid has a strong antioxidant effect, has a significant growth-promoting effect on animals, is cheap, has no toxic side effects, and has great use value in feed.
5. Application in pesticides
Rotenone is an insecticidal active substance extracted from the Roten plant. It has excellent contact killing and stomach poisoning effects on pests. It does not pollute the environment and the pests are not easy to develop resistance. It is an excellent insecticidal agent. agent. However, its efficacy is easily affected by environmental factors, is unstable in nature, and is susceptible to oxidative degradation and photolysis. Studies have found that using pyrogallic acid as an antioxidant and photoprotectant can prevent its degradation and extend the efficacy, and the degradation rate is inversely proportional to the dosage of pyrogallic acid. It well solves the problem of rotenone being unstable in the environment.
Using pyrogallic acid to react with 2,2-dimethylpropane and then reacting with methyl isocyanate to prepare dipiocarb, the total yield is 68%. Dimethocarb is a carbamate heterocyclic pesticide that is used for both health and agricultural production. It is a new variety of highly efficient and low-residue pesticides. It is in line with the national industrial policy for pesticide development and also makes full use of my country’s gallnuts. Resource pyrogallic acid, this process has high economic and social benefits.
6. Applications in other areas
The application of pyrogallic acid in other fields has also been reported. For example, liquid crystal ligands and some new imaging materials can be synthesized using pyrogallic acid as raw materials, and it is widely used as a flotation agent in metallurgy.
[References]
[1] Li Yuxin. Properties of pyrogallic acid and its preparation method[J]. Forest Products and Chemical Industry Communications, 1993 (4): 13-15.
[2] Du Yunping, Zhang Zonghe. Preparation methods and applications of pyrogallic acid[J]. Biomass Chemical Engineering, 2011, 45(1): 47-52.
[3] Li Lanying, Li Yuxin. Preparation and use of pyrogallic acid[J]. Hunan Chemical Industry, 1991, 21(4): 13-15.
[4] Editor-in-Chief Wen Huiliang. Chemical Additives. Nanchang: Jiangxi Science and Technology Press. 2009. Pages 618-619.
[5] Shen Panwen, Wang Jitao, editor-in-chief. Dictionary of Compounds. Shanghai: Shanghai Dictionary Publishing House. 2002. Page 552.
[6] Wu Shimin, Yin Delin, editor-in-chief. Concise Dictionary of Fine Chemicals. Shenyang: Liaoning Science and Technology Press. 1999.
[7] Practical Drug Handbook. Jinan: Shandong Science and Technology Press. 1999. Page 1274.

 微信扫一扫打赏
微信扫一扫打赏

