[Overview]
Idebenone (IDBN), whose chemical name is 6-(10-hydroxydecyl)-2,3-dimethoxy-1,4-benzoquinone, activates the wire pulling function Effect: It has an effect on improving brain function metabolism and brain dysfunction; at the same time, it increases the utilization rate of glucose in the brain and promotes the generation of ATP; it improves the metabolism of the neurotransmitter 5-tritonin in the brain, and has strong antioxidant and scavenging freedom. The role of base. It is clinically used to treat cerebral infarction, cerebral hemorrhage, brain dysfunction caused by sequelae of cerebral arteriosclerosis, low consciousness, mood disorders, language disorders and dementia.
[Physical and Chemical Properties]
Idebenone is a yellow-orange or orange crystal or crystalline powder, odorless, easily soluble in chloroform, methanol and anhydrous ethanol, easily soluble in ethyl acetate, and insoluble in n-hexane and water. , m. p is 53℃~55℃.
[Preparation method]
The side chain of idebenone is a 10-carbon straight chain with a hydroxyl group at the end. The raw materials of the parent compound are easily available. The key steps in the synthesis are the synthesis of the side chain and how to efficiently introduce the side chain into the parent compound.
1. Synthesis of side chains In the synthesis of idebenone, there are mainly 4 types of side chains (1) CH3COO(CH2)9COCI (2) CH3OOC(CH2)8COCI (3)[ROOC(CH2)8COH] 2O2R= CH3,C2H5 (4)[CH3COO(CH2)9CO]2O2 There are many studies on the synthesis of side chain 10-acetoxydecanoyl chloride (1). The raw materials are easily available, the method is simple, and the yield is high.
The synthesis method is shown in Figure 1:
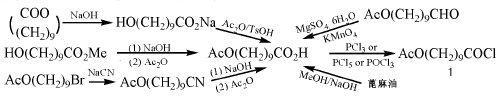
Figure 1 shows the synthetic route of 10-acetoxydecanoyl chloride
The most recommended synthesis method of the key intermediate 10-acetoxydecanoic acid is directly prepared from castor oil. When preparing compound 1 with 10-acetoxydecanoic acid, if phosphorus trichloride is used instead of sulfoxide chloride or phosphorus pentachloride, the generated chloride can be directly used in the next reaction without treatment. The reaction operation is simple and the product The quality is also good.
2. Directly introduce side chains to the parent (1) Use 3,4,5-trimethoxytoluene as the parent to introduce 10-acetoxydecyl or 9-alkoxycarbonyl through Freund-Crafts acylation reaction The two synthetic routes of nonanoyl are shown in Figure 2:
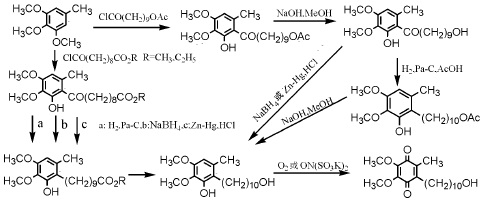
Figure 2 shows the synthesis of idebenone using Freund-Crafts acylation reaction
(2) Introducing 10-acetyl by reacting 2,3-dimethyl-5-methyl-1,4-benzoquinone with di(acetoxydecanoyl) peroxide in the presence of aluminum trichloride Oxydecanoyl, and then undergo hydrolysis treatment with NaOH-MeOH system to obtain the target product, or react with di(10-methoxycarbonylnonanoyl) peroxide to introduce 9-methoxycarbonyldecanoyl, and then reduce it with lithium aluminum hydride and Ferric chloride is oxidized to obtain the target compound.
The synthesis route is shown in Figure 3:

Figure 3 shows the synthesis of idebenone
The parent compound 2,3-dimethoxy-5-methyl-1,4-benzoquinone (Q0 for short) is an important intermediate in the synthesis of many ubiquinone compounds. Its main synthesis method is to use 1,2 , 3-phloroglucinol is used as the starting material and undergoes methylation and formylation reactions in sequence to obtain 2,3,4-trimethoxy-benzaldehyde, which is reduced to 2,3,4-trimethoxy-benzaldehyde through Clemenson reaction. Base-toluene, which is then oxidized by Freemason’s salt.
[Pharmacological effects]
1. Activate mitochondrial respiratory activity;
2. Improve brain energy metabolism in cerebral ischemia;
3. Improve glucose utilization in the brain and increase ATP production in the brain;
4. Inhibit the production of lipid peroxide by brain mitochondria;
5. Inhibit membrane disorders caused by lipid peroxidation of body membranes in the brain;
6. An effective antioxidant and scavenger of free radicals in brain cells;
7. It can effectively inhibit platelet aggregation and promote the release of 5-hydroxytryptamine.
【Security】
The acute toxicity of idebenone, intraperitoneal injection LD5o is 757-886mg/kg body weight, while subcutaneous and oral administration LD50>10000mg/kg body weight. For subacute toxicity, the oral non-toxic dose for rats is 500mg/kg/day. When the dose reaches 2500mg/kg/day, there are some toxic side effects. If you continue to observe for 5 weeks, these side effects will disappear naturally. Long-term toxicity: rats take 20 mg/kg/day orally for half a year, and dogs take 100 mg/kg/day for one year. It has no rheumatoid, carcinogenic or mutagenic effects.
[Pharmacokinetics]
Idebenone can be rapidly and evenly absorbed by the whole brain, with better absorption in the cerebral cortex, cerebellum, thalamus and auditory nucleus. The absorption rate of the brain in diseased parts is higher than that of the normal brain, and it can increase glucose in the brain. Utilization rate: The metabolism of idebenone is carried out through β-oxidation of its side chain and reduction of the quinone ring, and finally the reduced form is combined into sulfate ester and glucoside. In the human body, when 30 mg of idebenone is taken after meals, the peak time is 3.31 hours, the maximum plasma concentration is 290 μg/ml, and the elimination half-life is 7.69 hours. No prototype drug was detected in urine, and 24-hour urinary excretion was 32%. Conclusion Experiments and clinical applications have proven that idebenone has good safety and pharmacokinetic parameters, especially in terms of distribution, which is concentrated in the cerebral cortex, cerebellum, thalamus and auditory nerve nuclei, and the diseased brain tissue is higher than the normal brain tissue. .
[Purpose]
1. Idebenone is structurally similar to coenzyme Q10 and has goodAntioxidant activity, is a very effective antioxidant. It works through the parent quinone ring. Compared with coenzyme Q10, idebenone has a shorter side chain, no isoprene unit, and an alcoholic hydroxyl group is added at the end of the side chain. This structural feature not only does not reduce its anti- Its oxidative activity makes it easier to pass through biological membranes than Coenzyme Q10. Animal experiments show that the antioxidant activity of idebenone is about 100 times that of coenzyme Q10. Idebenone can eliminate various free radicals (including organic free radicals) such as N,N-diphenyl-N′-picrylhydrazyl free radicals, tyrosyl free radicals, hydrogen peroxide free radicals and peroxide free radicals. Nitrates, etc., which can prevent free radical-promoted lipid peroxidation. Antioxidant experiments show that the antioxidant activity of idebenone is 50% to 100% higher than that of vitamin E and fat-soluble vitamin E (Trolox). Idebenone can effectively inhibit lipid peroxidation that occurs in brain tissue homogenates, mitochondrial membranes and nerve cells due to oxidative stress, thereby protecting cells and mitochondria from oxidative damage. Idebenone can minimize the production of thromboxane by platelets and inhibit platelet aggregation, thereby maintaining the integrity of the blood vessel wall and its normal function.
2. Animal experiments show that idebenone can activate the respiratory activity of brain mitochondria, improve brain energy metabolism, and stimulate the synthesis of nerve growth factors. It is an energy metabolism improving drug. Idebenone improves mitochondrial dysfunction by activating the electron transport system and accelerating the generation of adenosine triphosphate (ATP), improving energy metabolism during cerebral ischemia and nervous system functional defects with vascular disease, thereby activating the function of the central nervous system. Studies have shown that even under the same cellular hypoxic conditions, idebenone still works equally well, preventing the production of free radicals and maintaining ATP energy levels required for normal cells, which is critical for brain and heart cells. As a brain metabolism activator, idebenone has a wide range of curative effects in the treatment of cerebrovascular diseases. It is non-toxic to the human body and has relatively few side effects.
3. Idebenone is an intelligence-promoting and anti-aging drug. It has antioxidant effects and can treat depression, low consciousness, and emotional disorders caused by sequelae of cerebral infarction, sequelae of cerebral hemorrhage, and cerebral arteriosclerosis. Speech barriers, etc. are curative. Idebenone is an analogue of Coenzyme Q10. In many ways, Idebenone can effectively replace Coenzyme Q10. Idebenone is clinically used to treat many degenerative diseases of the central nervous system related to oxidative stress, such as Parkinson’s disease, Alzheimer’s disease, multi-infarct dementia, and partial brain disease. Anemia, brain failure, etc., especially for the treatment of Friedreich’s ataxia (Friedreichataxia), idebenone can also be used for eye diseases caused by oxidation.
[Main reference materials]
[1]Chen Dongen. Research on the synthesis of idebenone[D]. Beijing University of Chemical Technology, 2007.
[2]Yang Chaowen, Yan Yunnan, Lei Ze, Fu Zhengqi, Mu Xiaoyun, Zhu Hongyou. Application overview and synthesis progress of idebenone [J]. Yunnan Chemical Industry, 2007(01):60-64+ 68.
[3] Yuan Xiangjie. Pharmacological effects and clinical application progress of idebenone [A]. Chinese Medical Association Neurology Branch. Compilation of papers of the 11th National Neurology Academic Conference [C]. Chinese Medical Association Neurology Branch:,2008:2.

 微信扫一扫打赏
微信扫一扫打赏

