[Background and Overview][1][2][3]
The molecular formula of nitrotoluene is CH3C6H5NO2. Toluene has a It repels electrons and is easier to nitrate than benzene. The mixed acid concentration used in the nitration of toluene is roughly the same as that in the nitration of benzene. A mixture of mononitrotoluene can be produced by reacting at 25-40°C. It consists of ortho body (63%), para body (33%) and a small amount of meta body (3%), which can be separated by cooling and vacuum distillation. Meta-nitrotoluene is a light yellow oily liquid or crystal with the smell of nitrobenzene. The molecular weight is 137.14, the relative density is 1.1571 (20℃), the melting point is 15.5~16.1℃, the boiling point is 232.6℃, and the refractive index is 1.5466 (20℃). Slightly soluble in water, soluble in ethanol, ether, chloroform and benzene. The parabody is a solid with a melting point of 52°C. O-Nitrotoluene is a yellow oily liquid with a nitrobenzene smell. It has stable α-type and unstable β-type. Relative density 1.1629 (20℃), melting point -3.17℃ (α type), -9.27 (β type), boiling point 221.7℃, refractive index 1.5450 (20℃), slightly soluble in water, soluble in ethanol, ether, chloroform, petroleum ether . The corresponding toluidines produced by the reduction of these three nitrotoluenes can be used as dye intermediates. In addition, the nitrotoluene mixture after separating the meta position is nitrated again to become a mixture of 2,4-dinitrotoluene (80%) and 2,6-dinitrotoluene (20%). It is then reduced to a mixture of diaminotoluenes, which are used as raw materials for toluene diisocyanate. It is used as raw material for dyes and in the manufacture of toluidine.
[Application][2][3][4]
O-Nitrotoluene can be used as raw material to synthesize other compounds such as o-nitrobenzaldehyde, o-nitrobenzoic acid, dinitrotoluene, etc. Examples of its application are as follows:
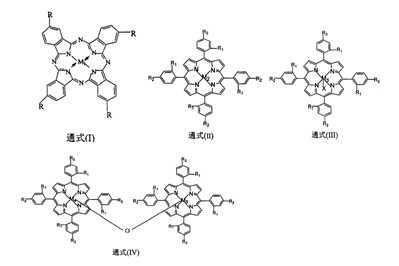
1. Used to prepare o-nitrobenzaldehyde. Using o-nitrotoluene as the raw material, metal phthalocyanines with the general formula (I) structure, mononuclear metalloporphyrins with the general formulas (II) and (III) or μ-oxygen binuclear metalloporphyrins with the general formula (IV) structure are selected. Any one of the phosphine as a catalyst, in the formula, M1, M2, M3, M4, M5 are transition metal atoms, M1=Fe, Co, Cu, Zn, M2=Fe, Mn, Co, Cu, Zn, M3= Fe, Mn, Co, M4 and M5 can be the same or different. When they are the same, M4=M5=Fe, Mn, Co. When they are different, M4=Fe, M5=Mn or M4=Fe, M5=Co, R can It can be carboxyl or hydrogen, R1 and R2 can be hydrogen, halogen, nitro, hydroxyl, alkoxy, the ligand As a solvent, in a 3.0-6.0 mol/L strong alkaline methanol solution, add 0.8-3.0 MPa oxygen, control the reaction temperature to 25-60°C, and the reaction time to 6-48 hours to obtain crude o-nitrobenzaldehyde. After separation and purification using conventional methods, o-nitrobenzaldehyde fine product is obtained. The structures of general formulas (I), (II), (III) and (IV) are as follows:
2. Used to prepare o-nitrobenzoic acid. Without adding any catalyst, o-nitrotoluene is used as the raw material, oxygen is the oxidant, and sodium hydroxide is the alkali. The reaction temperature is 25-85°C, and the reaction is carried out in the solvent alcohol or its aqueous solution for 5-72 hours. Process, separate and purify to obtain the o-nitrobenzoic acid. This method does not require a catalyst; the raw materials and solvents are cheap; the reaction temperature is moderate; the production is easy to control, the yield can be as high as 90%, and it is suitable for large-scale preparation and industrialization, and has broad application prospects.
3. Used to prepare dinitrotoluene. Using o-nitrotoluene as raw material, using fuming sulfur-fuming nitric acid nitration system, the nitration reaction was carried out at room temperature, and a dinitrotoluene mixture was obtained with high yield. The proportion of 2,4-DNT in dinitrotoluene mixtures can reach 75%. The nitration reaction is carried out at room temperature, which avoids the generation of trinitrotoluene and improves the safety of the nitration process. Specifically, it includes the following steps: the first step, add fuming sulfuric acid to the reactor, then add o-nitrotoluene dropwise, mix evenly, then add fuming nitric acid, and continue the reaction; the second step, after the reaction is completed, add the mixed solution Pour into crushed ice to precipitate the solid. After the ice cubes are completely melted, the solid obtained by suction filtration is washed with hot water, cooled, suction filtrated, and dried to obtain dinitrotoluene. Among them, the mass fraction of sulfur trioxide in fuming sulfuric acid in the first step is 5~25%; the dosage ratio of o-nitrotoluene, fuming sulfuric acid and concentrated nitric acid is 1 mol: 0.40~0.65 L: 1~1.05 mol ; In the first step, the reaction temperature is maintained at 5~30°C and the reaction time is 2~3h; in the second step, the mass fraction of 2,4-dinitrotoluene in the obtained dinitrotoluene is 72~75% ; The nitrification process adopts intermittent production or continuous production.
【Preparation】[7]
Toluene is nitrated with mixed acid to produce mixed nitrotoluene, which is mainly o-nitrotoluene (accounting for about two-thirds) and p-nitrotoluene (accounting for about one-third). The pure product can be obtained after separation . Add toluene into the reactor, cool it to below 25°C, add the prepared mixed acid (i.e. nitric acid 25-30%, sulfuric acid 55-58% and water 20-21%), and adjust the temperature not to exceed 50°C. Stir continuously for 1-2 hours, then let it stand for 6 hours. Separate the generated nitrotoluene, wash with water and alkali to remove unreacted toluene and aliphatic compounds. The composition of the crude nitrotoluene product is o-nitrotoluene 55-60 %, meta position 2-5%, counter position 35-40%. The yield is 90-95%. Use the difference between boiling point and melting pointThrough crude distillation and crystallization, each isomer can be separated. That is, the crude nitrotoluene is first subjected to vacuum distillation to separate most of the o-nitrotoluene, and the remaining fraction containing more p-nitrotoluene is separated by vacuum distillation, cooled, crystallized, and separated to obtain Finished product. High-boiling tar-like substances remain in the still. Meta-nitrotoluene is contained in the mother liquor after separation of the para-mer, and is obtained by distillation after repeated accumulation. The purity of ortho- and para-nitrotoluene can reach 98% and 99% respectively. The domestic process involves two pots connected in series, with the reaction temperature of the main pot being 40-45°C and the secondary pot being 50-55°C. The preparation of mixed acid is roughly similar, 26-28% nitric acid, 56-57% sulfuric acid, and 16-18% water. Raw material consumption quota: toluene (98%) 800kg/t, nitric acid (98%) 470kg/t, sulfuric acid (92.5%) 450kg/t, caustic soda (42%) 100kg/t.
[Main reference materials]
[1] Fine Chemical Dictionary
[2] She Yuanbin; Zhong Rugang; Fan Lili; Wu Shengzhou; Zhou Xiantai. Method for preparing o-nitrobenzaldehyde by bionic catalytic oxygen oxidation of o-nitrotoluene. CN200310121478.1, application date 2003-12-18
[3] She Yuanbin; Fang Kun; Li Guijie; Fu Haiyan. A method for preparing o-nitrobenzoic acid by oxygen oxidation of o-nitrotoluene without a catalyst. CN201611219737.8, application date 2016-12-26
[4] Lu Ming; Wang Pengcheng; Zhou Xinli; Yao Kai. Method for preparing dinitrotoluene by nitrating o-nitrotoluene in fuming sulfuric acid-nitric acid system. CN201310256194.7, application date 2013-06-26

 微信扫一扫打赏
微信扫一扫打赏

