Background and overview[1]
4-Bromobenzoylacetonitrile can be used as a pharmaceutical synthesis intermediate. It can be prepared from 3-bromobenzoic acid as the reaction raw material. The intermediate 3-bromobenzoic acid methyl ester is first prepared, and 4 is generated under the action of sodium cyanide. -Bromobenzoylacetonitrile.
Preparation[1]
Step 1: Methyl 3-bromobenzoate
To a mixture of 3-bromobenzoic acid (85.0 g, 423 mmol) in methanol (700 mL) was added dropwise thionyl chloride (151 g, 1.27 mol) under a nitrogen atmosphere at room temperature. The mixture was stirred at 62°C for 16 hours. Upon completion, the reaction was concentrated in vacuo to give a residue. The residue was washed with saturated sodium bicarbonate (100 mL) and extracted with dichloroethane (2 x 100 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo to give the title compound. 1HNMR (400MHz, CDCl3) δ=8.21 (s, 1H), 8.09-7.90 (m, 1H), 7.78-7.61 (m, 1H), 7.46 -7.21 (m, 1H), 3.97 (s, 3H).
Step 2: 4-bromobenzoylacetonitrile
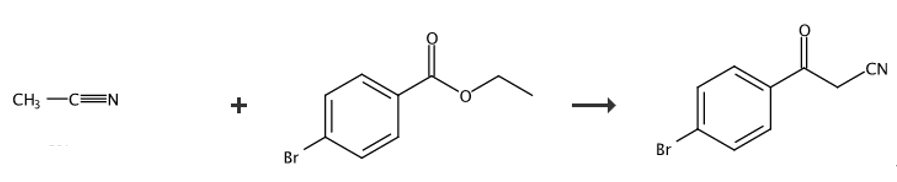
Under nitrogen atmosphere, add sodium hydride (6.05g, 151mmol) in portions to a mixture of acetonitrile (7.78g, 190mmol, 9.97mL) and anhydrous tetrahydrofuran (300mL) at room temperature, and then add 3-bromobenzene Methyl formate (25.0 g, 116 mmol) was added to the mixture, and the resulting mixture was heated to 77 °C and stirred for 2 hours. After completion, the reaction was cooled to room temperature, and hydrochloric acid solution (1N, 400mL) was added to the reaction. The aqueous layer was extracted with ethyl acetate (4 × 250mL), and the organic layer was washed with sodium bicarbonate (1.0L) and sodium sulfate. Dry and concentrate in vacuo to give the title compound 4-bromobenzoylacetonitrile. 1HNMR (400MHz, CDCl3) δ=8.07 (brs, 1H), 7.94-7.76 (m, 2H), 7.44 (t, J=7.6Hz, 1H ), 4.10 (s, 2H).
References
[1] WO2017156179 – 3-PHOSPHOGLYCERATE DEHYDROGENASE INHIBITORS AND USES THEREOF

 微信扫一扫打赏
微信扫一扫打赏

