Background and overview[1]
O-trifluoromethylstyrene is a commonly used monomer in the synthesis of polymer materials fluorinated polystyrene. So far, there are two methods for the synthesis of o-trifluoromethylstyrene reported in the literature. The first method is to use trifluoromethylphenylethyl alcohol as the raw material and potassium hydrogen sulfate as the dehydrating agent to perform a heating dehydration reaction. The second method is to use trifluoromethylphenylethyl alcohol as the raw material, use phosphorus pentoxide as the dehydrating agent in anhydrous benzene solvent to perform a heating dehydration reaction. Since o-trifluoromethylstyrene is unstable, current reporting methods are all in acidic conditions. When heated and dehydrated, self-polymerization of o-trifluoromethylstyrene will occur, resulting in a decrease in yield. Therefore, the synthesis of this compound has not yet yielded satisfactory results so far.
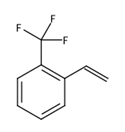
Preparation[1-2]
Report 1,
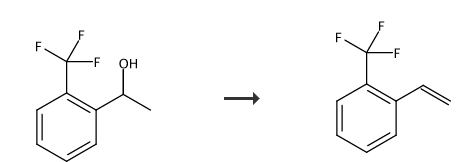
Add potassium dihydrogen phosphate (19g) and 3-trifluoromethylphenylethyl alcohol (190g) into the reaction bottle. Then heat to 100°C and stir for 12 hours. After distillation under reduced pressure, o-trifluoromethylstyrene (65-66°C/41mmHg) was obtained as a colorless liquid with a yield of 90% and a purity of 99.0% (GC).
Report 2,
Add KOSiMe3 (896 mg, 7 mmol, 3.5 equivalents) and tripalladium dibenzylideneacetone (58 mg, 0.1 mmol, 0.05 equivalents) into a 10 mL round-bottomed flask equipped with a magnetic stirring bar. and triphenylphosphine oxide (28 mg, 0.1 mmol, 0.05 equiv), filled with argon, and then the flask was charged with THF (4 mL), 2-bromotrifluorotoluene (450 mg, 2 mmol) and DVDS (500 μL, 2.2 mmol, 1.1 equivalent). The mixture was placed in a preheated oil bath and heated to reflux for 3 hours. After the completion of the reaction was determined by GC analysis, the mixture was cooled to room temperature, then filtered through a small silica gel pad (5g), the silica plug was rinsed with 50mL ether, and the organic layer was concentrated in vacuo and passed through column chromatography (silica gel, 30mmx140mm, pentane alkane; then RPC-18 silica gel, methanol/water 9:1) purified the crude product to obtain 172 mg (52%) o-trifluoromethylstyrene as a clear colorless oil, yield 172 mg (52%). 1HNMR: (500MHz, CDCl3) 7.66 (d, J=7.8, 1H, HC (7)), 7.63 (d, J=7.8, 1H, HC (4)), 7.52 (t, J=7.6, 1H, (HC (6))), 7.36 (t, J=7.7, 1H, HC (5)), 7.10 (ddd, J=17.3, 11.0 and 2.5, 1H, HC (2)), 5.75 (d, J=17.1, 1H, HtransC (1)), 5.42 (dd, J=11.0 and 1.2, 1H, HcisC (1). 13CNMR : (125MHz, CDCl3) 137.0 (C (3)), 133.3 (C (4)), 132.1 (C (2)), 127.7 (C (5)), 127.3 (C ( 6)), 125.8 (q, C (9)), 125.6 (C (8)), 123.4 (C (7)), 118.2 (C (1)). IR: 3083 (w), 2886 ( m), 2801 (m), 1876 (w), 1611 (s), 1555 (w), 1522 (s), 1480 (m), 1444 (m), 1407 (w), 1351 (s), 1228 ( m), 1187 (s), 1166 (s), 1129 (w), 1061 (m), 990 (m), 946 (m), 887 (m), 821 (s), 751 (m), 691 ( w).
References
[1]CN101781161-A preparation method of o-trifluoromethylstyrene compounds
[2] Denmark S E , Butler C R . Vinylation of Aromatic Halides Using Inexpensive Organosilicon Reagents. Illustration of Design of Experiment Protocols[J]. Journal of the American Chemical Society, 2008, 130(11):p.3690-3704 .

 微信扫一扫打赏
微信扫一扫打赏

