Henkel, a leading manufacturer of adhesive solutions for the medical industry, has launched a new medical-grade light-curing adhesive designed for body-worn devices. This new product does not contain IBOA (isobornyl acrylate) or other known skin sensitizing monomers. The adhesive is also formulated to comply with the EU MDR (EU Medical Device Regulation)
The 2017 regulations on the use of CMR (carcinogenic, mutagenic or toxic to reproduction) substances in medical devices make it a reliable option for new designs.

(Loctite WT 3001 and Loctite WT 3003 are new medical-grade light-curable adhesives for housing, sealing and bonding applications that have been tested to the ISO10993 protocol.)
Two newly developed adhesive products, Loctite WT 3001 and Loctite WT 3003, have passed ISO
10993 standard testing provides users with a high level of assurance in terms of performance and safety. Both products have also passed skin allergy testing, making them suitable for a variety of medical applications including wearable devices.
As the demand for medical devices and wearables grows, the need for safe and reliable adhesive solutions becomes even more critical. Henkel’s new light-curable adhesive for wearable devices provides an excellent bonding solution for medical devices, ensuring a strong and durable bond while containing no known skin-sensitizing monomers such as isobornyl acrylate (IBOA) ). The industry believes that IBOA can cause skin allergies in patients when used in wearable devices.
“At Henkel, we are committed to providing our customers with innovative adhesive solutions that meet the highest safety standards,” said Philipp, Vice President Europe, India, Middle East and Africa and Global Head of the Medical Industry at Henkel Industrial Electronics.
Loosen said, “With the launch of our new medical-grade light-curable adhesive for wearable devices, we are meeting the medical industry’s growing demand for safe, effective adhesives. Our adhesives are ISO certified
10993 test, its formula takes into account patient comfort and safety, ensuring its suitability for medical device bonding and medical wearable devices. ”
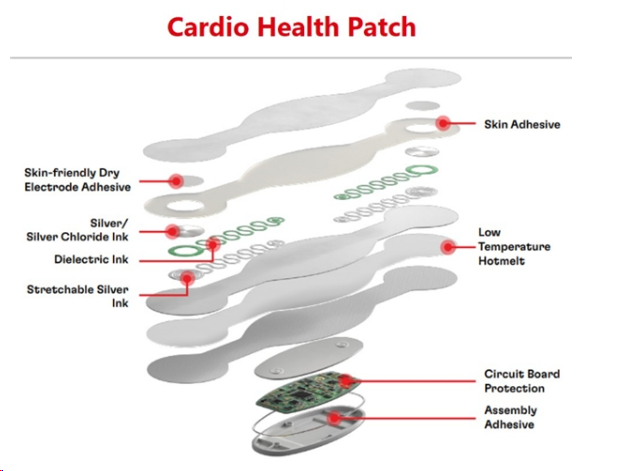
(Henkel offers a broad portfolio of medical wearables, such as heart health patches.)
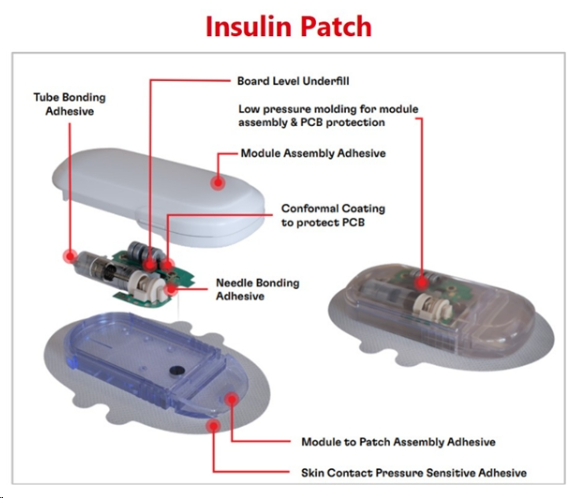
(Henkel offers a broad portfolio of medical wearable devices, such as those used in insulin pumps.)

(Henkel offers a broad portfolio of medical wearables for applications such as continuous glucose monitoring.)
Henkel’s light-curable adhesives for wearable devices offer multiple benefits for medical applications. Its light-curing properties enable fast and efficient bonding, reducing production time and increasing productivity. The adhesive also provides excellent adhesion to a variety of substrates commonly used in medical and wearable devices, ensuring strong bonding and sealing properties remain after drops, impacts, or bathing.
Additionally, the adhesive is formulated to comply with U.S. Food and Drug Administration and EU Medical Device Regulation recommendations for wearable medical devices regarding allergenic and CMR ingredients. Henkel is committed to meeting regulatory requirements and providing customers with safe and reliable adhesive solutions. Henkel’s focus on removing all known skin-sensitizing monomers further reduces the risk of skin sensitization, making the adhesive suitable for long-term skin contact in medical applications.
With the trend of integrated and connected devices, medical wearables are expected to integrate advanced technologies with a data-driven approach into the healthcare industry. Henkel offers a broad portfolio of materials solutions for medical wearables, including low-pressure injection molding, skin-contact pressure-sensitive adhesives, structural bonding adhesives, circuit board protection and printed circuits for biosensors.

 微信扫一扫打赏
微信扫一扫打赏

