Background and overview[1]
2-Methoxy-4-(2-aminoethyl)phenol hydrochloride can be used as a pharmaceutical synthesis intermediate and can be prepared by the reaction of vanillin and nitromethane. 2-Methoxy-4-(2-aminoethyl)phenol hydrochloride can be used to prepare tetrahydropalmatine derivatives for the prevention and treatment of hyperlipidemia, hypercholesterolemia, and hypertriglyceridemia. , hepatic steatosis, type II diabetes, hyperglycemia, obesity or insulin resistance and metabolic syndrome.
Preparation[1]
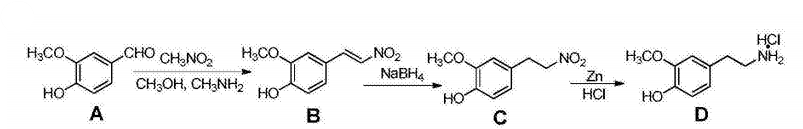
Put vanillin A (30.4, 200 mol) and nitromethane (16.1 ml, 300 mmol) into methanol in sequence, and add 33% methylamine alcohol solution (1.5 ml). Stir the reaction at 50°C for 2 hours. The solution is homogeneous and gradually changes from colorless to dark brown. A large amount of yellow solid appears in 1.5 hours. After TLC detects that the reaction is complete, let it cool and filter with suction. Yellow solid B (33.6g, 172.3mmol) washed with a small amount of methanol. Add B (33.6g, 172.3mmol) prepared in the previous step to 1,4-dioxane (500mL), stir vigorously, and turn into a turbid liquid for later use. In addition, put ethanol (75mL), 1,4-dioxane (300mL), and NaBH4 (32.0g, 845.9mmol) into the eggplant-shaped flask in sequence, and add the reserved suspension dropwise while stirring. liquid, add it in about 1 hour. Stir at room temperature overnight. The solution gradually turns orange-red. Add 250 ml of ice water to the system the next day, adjust the system to weak acidity with glacial acetic acid, transfer it to a separatory funnel, extract with ethyl acetate, wash the organic layer with water and saturated brine, remove the ethyl acetate under reduced pressure, and obtain a brown-red color Oil C (32.0g, 162.4mmol). Add C (8g, 40.6mmol) prepared in the previous step to ethyl acetate, concentrated hydrochloric acid, heat to 70°C, slowly add activated zinc powder (35.0g) under vigorous stirring , 546.5mmol), after the addition is completed, slowly cool to 50°C, react for 3 hours, maintain vigorous stirring during the reaction, and detect by TLC. After the reaction is complete, cool to room temperature, filter, remove excess zinc powder, dry the filtrate over anhydrous sodium sulfate, and then filter and collect The filtrate was concentrated to obtain oily substance 4 (6.7g, 40.0mmol). Add 4M/L hydrochloric acid-methanol solution to oily substance 4, shake it, and place it in a -20°C refrigerator for 2 hours. The solid was precipitated and suction filtered to obtain 2- Methoxy-4-(2-aminoethyl)phenol hydrochloride 6g (reaction yield: about 72%). 1HNMR (400MHz, DMSO) δ9.02 (s, 1H), 6.85 (d, J = 8.2Hz, 1H), 6.69 (d, J = 2.0Hz, 1H), 6.62 (dd , J=8.1, 2.0Hz, 1H), 3.73 (s, 3H), 2.95–2.91 (m, 2H), 2.76–2.72 (m, 2H).
References
[1]CN201410475835.2 Tetrahydropalmatine derivatives and their applications

 微信扫一扫打赏
微信扫一扫打赏

