Background and overview[1]
3,4,5-Trimethoxybromobenzene can be used as a pharmaceutical synthesis intermediate. It can be prepared by the reaction of 5-bromo-1,2,3 trihydroxybenzene and dimethyl sulfate, or it can also be prepared from 4-bromo-2 , 6-dimethoxyphenol was prepared as the reaction raw material.
Preparation[1-2]
Option 1,
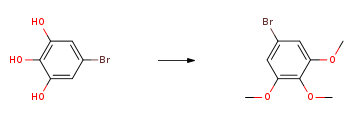
Cool the mixture of 5-bromo-1,2,3 trihydroxybenzene (1.3g, 5.63mmol), 1M NaOH (14mL) and dimethyl sulfate (800μL, add 16.89mmol) to 10°C. The mixture was heated to reflux for 3 hours, then another portion of dimethyl sulfate (800 μL) was added. The mixture was heated under reflux for another 3 hours, the mixture was cooled overnight, and the gray solid was filtered off, then dissolved in 50 mL of diethyl ether, with 5% NaOH-H2O, water, brine Washed, then dried (MgSO4) and concentrated to give 3,4,5-trimethoxybromobenzene (1.39g, 76%). 1HNMR (CDCl3) δ6.72 (s, 2H), 3.85 (s, 6H), 3.82 (s, 3H); m/z. 13CNMR (CDCl3) 136.67, 137.11, 115.92, 108.07, 60.58, 56.05.
Option 2,
Cool a mixture of 74g (0.32mol) 4-bromo-2,6-dimethoxyphenol and 32g (0.8mol) NaOH in 850ml H2O to 10° C, then add 45 ml (0.48 mmol) of dimethyl sulfate, reflux the mixture for 3 hours, and then add an equal amount of dimethyl sulfate (0.96 mol in total). The mixture was refluxed for another 3 hours. After cooling overnight, the gray product solidified and was filtered out and dissolved in 1.21 ether. The ether solution was filtered to remove insoluble impurities, and was sequentially treated with 5% NaOH solution (200ml), water (2 × 200mL). ) and washed with brine (200 mL). The ether phase was dried over Na2SO4 to obtain an off-white solid, which was recrystallized in hexane (300 ml) to obtain 62.3 g (79%) of 3,4 , 5-Trimethoxybromobenzene.
References
[1]US2003176478
[2] CN01819067.7 Ortho-substituted chiral phosphines and trivalent phosphonates and their use in asymmetric catalytic reactions

 微信扫一扫打赏
微信扫一扫打赏

