Background and overview[1]
Methyl 2-methoxy-5-sulfonamide benzoate is an important intermediate for the antipsychotic drugs sulpiride and levosulpiride. Its synthesis currently uses salicylic acid through methylation, chlorosulfonation, The product is obtained by amination and esterification. The process route is long and a large amount of three wastes are generated at each step, especially high chemical oxygen demand (COD), high salt, high ammonia nitrogen, mixed wastewater, etc., which makes treatment difficult and extremely costly. High, which seriously restricts the large-scale industrial production of this product.
New preparation method[1]
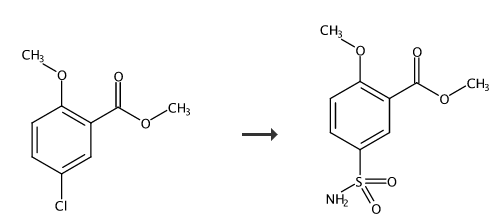
Into a 1000ml reaction bottle equipped with a reflux device, 300g tetrahydrofuran, 50g (0.25mol) methyl 2-methoxy-5-chlorobenzoate, 1.8g cuprous bromide (0.0125mol), 25.7g (0.25 mol) sodium aminosulfinate, raise the temperature to 65°C, and maintain it at this temperature for 12 hours. After the insulation is completed, add 2 grams of activated carbon to the reaction solution and heat it to filter. The filtrate is concentrated to dryness under reduced pressure and dried under vacuum at 60°C. Obtained, 57.9g (0.236mol) of 2-methoxy-5-sulfonamidobenzoic acid methyl ester as white crystalline powder was obtained, with a yield of 94.5% and a content of 99.51% (HPLC). Among them, HPLC detection conditions: mobile phase: 700 ml of water; 200 ml of methanol. Detection wavelength: 240nm, flow rate 1.0ml/min, sample 0.01g, diluted to 25ml with mobile phase, injection volume 5μl.
Apply[2]
2-Methoxy-5-sulfonamide benzoic acid methyl ester can be used to prepare sulpiride, sulpiride (sulpiride), chemical name is N-[(1-ethyl-2-pyrrolidinyl)methyl]-2 -Methoxy-5-(sulfamoyl)benzamide; synthesized by the French in 1967. Due to its ability to treat mental disorders and few adverse reactions, it has been used clinically at home and abroad for many years. The specific reaction steps are: add 49g of 2-methoxy-5-sulfonamide benzoic acid methyl ester and 26.5g of (S)-1-ethyl-2-aminomethyltetrahydropyrrolidine into the reaction bottle, and protect it with nitrogen. React at 90-100°C for 5 hours. When the reaction is completed, cool to 80°C, add 50g of ethanol, stir and reflux for 10 minutes, cool to 5°C and stir for 2 hours, filter, wash with ethanol, and dry at 65°C. The yield is 93.8% and the purity is 99.2%.
References
[1]CN201511010526.9 A method for preparing 2-methoxy-5-sulfonamide benzoic acid methyl ester
[2]CN201210445711.0 A synthesis and post-processing method of sulpiride or its optical isomer

 微信扫一扫打赏
微信扫一扫打赏

