Background and overview[1]
2-Hydroxy-5-chlorobenzophenone can be used as a pharmaceutical synthesis intermediate. It can be prepared from 4-chlorophenyl benzoate as the reaction raw material, or by the reaction of substituted salicylic acid and arylboronic acid. .
Preparation[1-2]
Report 1,
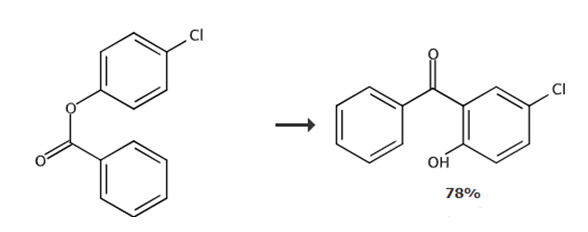
Add 4-chlorophenyl benzoate (23.3 g, 100 mmol) and anhydrous aluminum chloride (16.7 g, 125 mmol, 1.25eq) into a 250 ml round-bottomed flask. The melted reaction mixture was slowly stirred at 160-180°C for 3 hours. The mixture was cooled to room temperature. Quench the mixture with ice-cold water (170 mL) and 10% aqueous hydrochloric acid solution (85 mL), add dichloromethane (200 mL) to the mixture, and shake well until completely dissolved. Transfer the solution to a separatory funnel, separate the aqueous layer from the organic layer, and extract twice more (2×200 mL). The combined organic layers were concentrated under reduced pressure to approximately 150 mL. Transfer the mixture to a separatory funnel and extract the mixture with 2.5% sodium hydroxide solution (4×250 mL). Acidify the combined alkaline aqueous layer with 10% sulfuric acid (pH≈1, approximately 300 mL), filter out the crude precipitate, and dry the filtrate in air. After purification with activated carbon, the crude crystals were crystallized from methanol to obtain 2-hydroxy-5-chlorobenzophenone.
Report 2,
Dissolve a mixture of substituted salicylaldehyde (0.2 mmol) and arylboronic acid (0.4 mmol) in DMF (2 mL). Add [Cp*RhCl2]2 (4.9 mg, 4 mol%) and Cu(OAc)2 (72.8 mg, 0.4), The reaction was carried out in a sealed tube at 80°C for 8 hours. The reaction mixture was diluted with ethyl acetate (20 mL) and washed with H2O (310 mL). The organic layer was washed with anhydrous Na2SO4dry. The crude product was loaded onto a silica gel column and flashed with 5-10% ethyl acetate in petroleum ether to give 2-hydroxy-5-chlorobenzophenone.
References
[1] Series of high spin mononuclear iron(III) complexes with Schiff base ligands derived from 2-hydroxybenzophenones
[2] Rh(III)-catalyzed aldehyde C-H bond functionalization of salicylaldehydes with arylboronic acids

 微信扫一扫打赏
微信扫一扫打赏

