Background and overview[1]
4-Heptyl-4′-cyanobiphenyl is a kind of liquid crystal materials (LCD). Liquid crystal material is a new type of high-performance special material developed in the 1970s. Its mesogenic phase is an intermediate phase between the three-dimensional order of the solid state and the randomness of the liquid state. It is a Orientation ordered fluid.
Biphenyl-type liquid crystal has the advantages of good optical and chemical stability, and adjustable physical constants such as anisotropy, viscosity and birefringence. It is one of the liquid crystal materials widely used in the modern electronics industry. Among the currently widely used biphenyl liquid crystal materials, 4-cyanobiphenyl and 4-cyanoterphenyl compounds account for a considerable proportion.
Preparation[1]
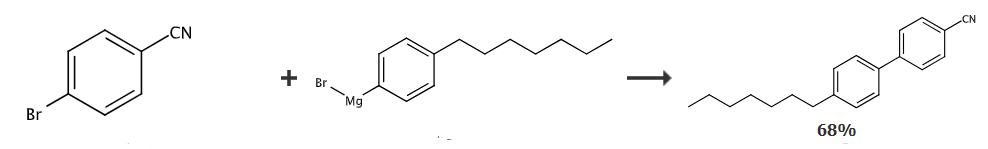
Under nitrogen protection, combine 20 mmol of 4-bromobenzonitrile, 0.2 mmol of palladium acetate, 0.24 mmol of Ph3P and 3 mmol of zinc bromide Dissolve in 30 ml of THF, and then add dropwise 50 ml of Grignard reagent prepared from 20 mmol of 4-n-heptylbromobenzene. After the dropwise addition is completed, react at room temperature for 2 hours. Afterwards, the reaction solution was cooled in an ice-water bath, and then 50 ml of 1% mass dilute brine solution was added dropwise. After the dropwise addition, stir for 30 minutes, separate the organic phase, and extract the aqueous phase twice with 50 ml of ethyl acetate, and combine the organic phases. , and then washed twice with 50 ml of water, and then the solvent was evaporated in a rotary evaporator to obtain the crude product. The crude product was recrystallized from methanol and decolorized with activated carbon to obtain pure 4-heptyl-4′-cyanobiphenyl. Yield: 68.2%.
1H NMR (DCCl3, 400MHz): δ 7.69-7.63 (m, 4H), 7.52-7.48 (m, 2H), 7.29-7.27 (m, 2H), 2.65 (t, J = 8 Hz, 2H), 1.66-1.63 (m, 2H), 1.35-1.27 (m, 8H), 0.90-0.87 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 145.6, 143.8, 136.4, 132.5, 129.1, 127.4, 127.0, 119.0, 110.5, 35.6, 31.8, 31.4, 29.2, 29.1, 22.6, 14.1.
References
[1] [Invented in China, authorized by China] CN201110417782.5 A method for synthesizing cyanobiphenyl liquid crystal material

 微信扫一扫打赏
微信扫一扫打赏

