Background and overview[1]
4-Bromobenzenesulfonamide can be used as a pharmaceutical synthesis intermediate. It can be prepared by the reaction of 4-bromobenzenesulfonyl chloride and ammonia water, or it can be prepared in one step from p-bromobenzene tetrafluoride boron diazonium salt.
Preparation[1-2]
Method 1:
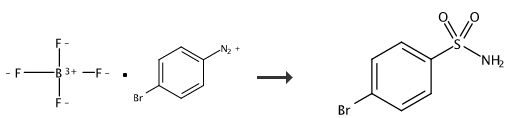
Under nitrogen protection, p-bromobenzene tetrafluoride boron diazonium salt (338.5mg, 1.25mmol), NaN3 (32.5mg, 0.5mmol), PPh3(157.4mg, 0.6mmol), Na2S2O5 (190.1mg, 1.0mmol), TBAB (241.7mg, 0.75mmol) and MeCN/H2O=2/1 (1mL) were added to the Schlenk reaction tube. After the reaction was stirred at 80°C for 12 hours, it was lowered to room temperature, 10 mL of water was added to the system to dilute, and then ethyl acetate (10 mL*3) was added for extraction, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain a white solid. 4-Bromobenzenesulfonamide (70%). 1HNMR (400MHz, d6-acetone) δ7.83 (d, J=8.6Hz, 2H), 7.76 (d, J=8.6Hz, 2H), 6.71 (s, 2H); 13CNMR (100MHz, d6-acetone) δ144.4, 132.9, 128.9, 126.7; IR (KBr) ν3329, 3239, 3117, 1575, 1391, 1310, 1148, 1091, 818, 742, 613cm -1; HRMS(EI)forC6H6NO2SBr Calculated:234.9303, found:234.9305.
Method 2:

Add ammonia water (1.0 equivalent) into the round-bottomed flask, add DCM and triethylamine (2.0 equivalent), stir for 30 minutes, add 4-bromobenzenesulfonyl chloride (1.2 equivalent) and continue the stirring reaction at room temperature overnight. After TLC detects that the reaction is complete, add water and DCM to the concentrated solvent for extraction three times, combine the organic phases, dry over anhydrous Na2SO4, and concentrate to obtain compound 4-bromo. Benzenesulfonamide.
Apply[1]
4-Bromobenzenesulfonamide can be used to synthesize 4-(5-aldehyde-furan)benzenesulfonamide: compound 4-bromobenzenesulfonamide (1.0 equivalent), 5-aldehyde-furan-2-boronic acid ( 1d, 1.5 equivalents), anhydrous sodium carbonate (2.0 equivalents) and bistriphenylphosphine palladium dichloride (0.1 equivalents) were dissolved with MeCN:H2O=2:1, and after replacing the argon gas three times, the temperature was raised to 80°C for reaction. 2h, after TLC detects that the reaction is complete, cool to room temperature, filter out the insoluble catalyst with diatomaceous earth, concentrate under reduced pressure, add water, extract with ethyl acetate, concentrate, and obtain 4-(5-aldehyde-furan) by column chromatography Benzenesulfonamide.
References
[1]CN201810015576.3 An acrylamide derivative and its preparation method and application
[2]CN201811335644.0 Sulfonamide compounds and their synthesis methods and applications

 微信扫一扫打赏
微信扫一扫打赏

