Background and overview[1]
Phthalocyanine is a very important industrial raw material, which was first used as a color filler in pigments, coatings, inks and paints. Because phthalocyanine also has photoconductive properties, nonlinear optical properties and other characteristics, it has been widely used in phototherapy, optical storage and other fields in recent years. The amino group is a strong electron-donating group and is easy to form acidic ionic compounds, so amino-substituted phthalocyanines are a very promising class of compounds. Phthalonitrile is an important intermediate in the preparation of phthalocyanine, so the preparation of amino-substituted phthalonitriles is very important.
4-Aminophthalonitrile is a typical representative of phthalonitrile, which is usually produced by catalytic hydrogenation under high pressure. However, this method has the following problems: First, because amino-substituted aromatic compounds are easy to oxidize and are not suitable for long-term storage, it is best to prepare them for immediate use. However, in many cases, it is often not possible to use an autoclave for production. Conditions, so it is difficult to make them for immediate use. At present, most of these compounds in our country are imported from abroad. Secondly, using high-pressure catalytic hydrogenation to produce 4-aminophthalonitrile has high production costs, which keeps the price of this type of product high.
Preparation[1-2]
Method 1: Synthesis of 4-aminophthalonitrile:

Dissolve 4-nitrophthalonitrile in 50mL methanol and 20mL hydrochloric acid, add iron powder (6g, 107mmol) in batches; after refluxing for 5 hours, the crude product is evaporated under reduced pressure and the solvent is evaporated to obtain the crude product. After separation by column chromatography (eluent: dichloromethane/ethyl acetate, 1/1), a white solid was obtained with a yield of 62%.
Method 2: The synthesis process of 4-aminophthalonitrile is as follows:
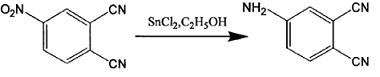
The specific synthesis steps are: gradually add 4 grams of 4-nitrophthalonitrile to an absolute ethanol solution in which 27 grams of stannous chloride dihydrate is dissolved, and stir the reaction at 70°C under nitrogen protection. After 1 hour, the reaction system became a homogeneous solution, cooled, mixed with a large amount of crushed ice, and gradually added saturated sodium bicarbonate solution until pH = 7.5. Filtered to obtain a white precipitate, dried, extracted with ethyl acetate, and rotary evaporated to obtain a yellow solid. The product was obtained by column chromatography. Yield: 85%. Melting point: 179-181℃. Mass spectrum: 143, elemental analysis C8H5N3 measured values (calculated values): C65.8 (67.1), H3.1 (3.5), N29.0 (29.4), proving that the product obtained is 4-aminophthalonitrile.
Main reference materials
[1] CN109897047-Soluble copper phthalocyanine and its preparation method and application
[2] CN1446797-A synthesis method of amino-substituted phthalonitrile

 微信扫一扫打赏
微信扫一扫打赏

