Background and overview[1][2]
1,2-Dibenzoylethane is an organic intermediate. It has been reported in the literature that 1,2-bibenzoylethane can be prepared in one step from racemic allyl secondary alcohol (±).
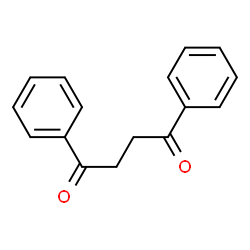
Preparation[1-2]
Report 1,
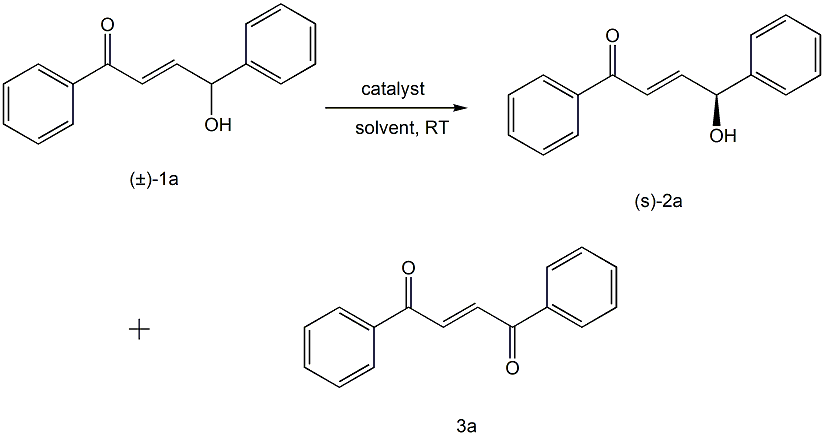
Using racemic allyl secondary alcohol (±)-1a as raw material, under the catalysis of chiral binaphthol potassium salt, the chiral allyl secondary alcohol 2a and 1,2-alcohol are reacted in an organic solvent. Benzoylethane 3a. The optimization results of reaction conditions are as follows:
Reaction conditions: (±)-1a (0.1mmol), catalyst (0.01mmol) reacted in solvent (1.0mL) at room temperature for 3 hours.
Report 2,
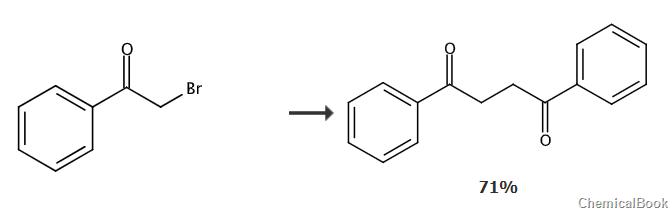
Dissolve benzoyl bromide (11.9g, 59.9mmol) and sodium hydroxymethanesulfinate dihydrate (9.24g, 59.9mmol) in dry DMF (90mL), and bring the resulting suspension to room temperature. Stir for 24 hours. Water (250 mL) was added and the white solid was collected and washed with water. The white solid was dissolved in chloroform (200 mL) and washed with 1 M NaOH (40 mL × 3). The organic layer was dried over anhydrous Na2SO4, concentrated, and dried under vacuum to obtain 1,2-bibenzoylethane (5.05g, 71%), which was White solid.
Apply[2]
1,2-Dibenzoylethane can be used to prepare multi-substituted pyrrole, which is a key structure and is commonly found in biologically active natural products and pharmaceuticals.
Preparation of 2,5-diphenyl-1H-pyrrole:
Combine 1,2-bibenzoylethane (5.05g, 21.2mmol) and ammonium acetate (9.80g, 127mmol)
Dissolve in acetic acid (140 mL) and reflux the resulting solution for 20 hours. After cooling, the reaction mixture was poured into ice water. The solid formed was collected, washed with water and dried to give 2,5-diphenyl-1H-pyrrole as a white solid (4.45 g, 96%).
Main reference materials
[1][Chinese invention] CN201910287141.9 A method for synthesizing chiral allyl secondary alcohols
[2]FromOrganicLetters,21(17),6972-6977;2019

 微信扫一扫打赏
微信扫一扫打赏

