Background and overview[1]
Ritadronic acid and its esters are pharmaceutically useful compounds that mainly act on the central nervous system. Ritedronic acid is a drug used to treat ADHD in children. It can be obtained by catalytic reduction of α-aryl-α-pyridin-2-yl-acetamide followed by hydrolysis of the resulting piperidinoacetamide, or by converting α-aryl-α-pyridin-2-yl – Obtained by catalytic reduction of acetates or esters.
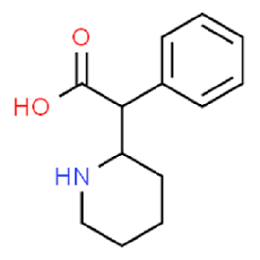
Preparation[1]
Preparation of Riteridronate:
Step 1: Hydrogenation
Put 20g of 2-pyridyl-phenylacetamide and 70mL of acetic acid into a pressurized reactor, bubble nitrogen into it and add 2g of 5% Rh/C, and hydrogenate at 15 bar and 50-55°C. After approximately 5/6 hours, the catalyst was filtered off and the solution concentrated under reduced pressure. The residue was diluted with 20 mL of water and added dropwise to the potassium hydroxide solution (pH>11). The precipitated solid was filtered and used in a subsequent step in its moist form.
Step 2: Isomerization
The wet product from step 1 was suspended in 36 mL of water and 19.24 g of 90% potassium hydroxide was added. The resulting white suspension was heated at 95-105°C for 6 hours. The mixture was then cooled to 0-5°C, filtered and washed with water. The resulting solid is vacuum dried or used in a subsequent step in its moist form.
Step 3: Hydrolysis
Put 20 g of the compound obtained in step 2 suspended in 73 mL of water into a 250 mL round-bottomed flask equipped with a magnetic stirrer, thermometer, condenser and dropping funnel and cooled with an ice bath. 27 mL of 98% sulfuric acid was added dropwise to the suspension. The mixture was heated to 80-85°C with stirring to complete hydrolysis of the amide (typically 8 hours), then the solution was cooled to room temperature and poured into 350 mL of water. 1.2 g of charcoal was added to the solution and kept stirring for 30 minutes, then filtered and washed with 30 mL of water. The pH of the solution was then adjusted to 6.0-6.2 with 30% NaOH. The resulting suspension was stirred at room temperature for 30 minutes and then filtered.
The resulting solid was washed with water and dried under vacuum at 50°C overnight. Yield: 10-15g Ritidronic acid, purity higher than 99.0%.
Main reference materials
[1] CN200580043121.3 Preparation of α-aryl-α-piperidin-2-yl-acetamide and its acidolysis method

 微信扫一扫打赏
微信扫一扫打赏

