Background and overview[1-2]
Chloroacetylcatechol is a key intermediate in the synthesis of carbachol. Carbacol, also known as Anluoxue and Adrenochrome Bicarbazide Sodium Salicylate, is mainly suitable for bleeding caused by increased capillary permeability.
Preparation[1]
1 Synthesis principle of chloroacetylcatechol
The original production process uses monochloroacetic acid and phosphorus oxychloride as auxiliary raw materials to produce chloroacetylcatechol. These two reactants are toxic substances compulsorily controlled by the public security department, and phosphorus oxychloride is easily decomposed into phosphoric acid and Hydrogen chloride has high requirements on the working environment. Hydrogen chloride is an acidic gas and is highly corrosive to equipment and can easily cause equipment damage. This study optimized the experimental raw materials and found by reviewing the literature that chloroacetyl chloride in acid halide compounds is highly active and easy to interact with. Aromatic hydrocarbons are combined, and its molecular structure meets the requirements of subsequent reactions, so chloroacetyl chloride is used as a substitute for monochloroacetic acid and phosphorus oxychloride. Sun Yaoran et al. used catechol and chloroacetyl chloride to perform the acylation reaction on the benzene ring Chloroacetylcatechol was prepared, and its synthesis reaction route is shown in Figure 1.
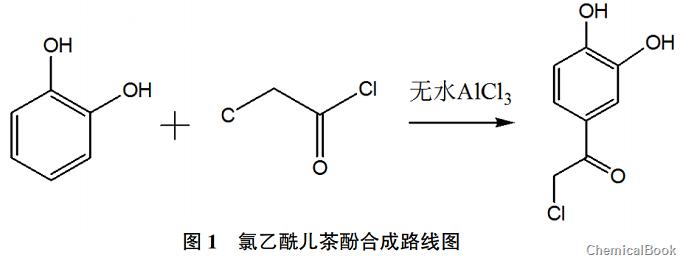
2 Synthesis operation of chloroacetylcatechol
In a dry three-necked flask equipped with a reflux condenser and stirring device, add a certain amount of 1,2-dichloroethane and control the solution temperature to 10-15°C. Pour a certain amount of anhydrous aluminum trichloride powder into the three-necked flask, turn on the stirring device, and continue stirring for 10-15 minutes to make the aluminum trichloride evenly dispersed in the organic solvent. Take a certain amount of catechol, slowly add it to the mixed solution, and continue stirring for 30 minutes. Maintain the system temperature at 10-15 °C during the process. Measure a certain amount of chloroacetyl chloride and pour it into a constant pressure dropping funnel. , add dropwise to the mixed solution at a rate of 1 drop/s. After the addition is completed, slowly increase the temperature to a certain temperature, continue stirring, and maintain a certain reaction time. After the reaction is completed, pour the product into an appropriate amount of ice water, and control the temperature at 10-15 °C, and stir for 30 min to precipitate a solid, which is then suction filtered, washed with water several times until neutral, and dried to obtain light gray powdery solid chloroacetylcatechol.
Apply[2]
Used to synthesize carbacrine. The steps are as follows:
1) Synthesis of adrenaline hydrochloride
Add methylamine solution into the reaction kettle, control the temperature below 10°C, add the product of step 1, stir after addition, raise the temperature to 35 ~ 40°C and keep the reaction for 4-5 hours, take samples for in-process control; after passing the in-process control, cool down to 0~10℃, centrifuge, take out the solid and add it to the kettle containing absolute ethanol, add hydrochloric acid to adjust the pH to 6.0~7.0, stir and centrifuge, take out the solid and add absolute ethanol to stir, add an appropriate amount of hydrochloric acid to form a salt, and control the temperature <45℃, after addition, cool to 0~10℃, stir, centrifuge, and dry to obtain adrenaline hydrochloride;
2) Synthesis of adrenaline
Add adrenaline hydrochloride and water into the pretreatment kettle, stir and dissolve, then add activated carbon for decolorization at 30 to 50°C, filter, transfer the mother liquor to the hydrogenation kettle, add sodium borohydride, control the temperature below 25°C, and add The pH of the final system is 9-10, react at 20-40°C for 1 to 2 hours, adjust the pH with hydrochloric acid to 4.0-5.0, cool to 0-5°C, and obtain an aqueous epinephrine solution. Without treatment, directly transfer to the oxidation and condensation process and enter the next step of the reaction;
3) Synthesis of carbacryl
Add the hydrogenation mother liquor into the kettle, add the filtered aqueous solution containing potassium ferricyanide and sodium bicarbonate at 0-5°C, stir, then add the filtered aqueous solution containing semicarbazide hydrochloride and potassium acetate, and stir at room temperature for 2 ~3h, let stand, centrifuge, take out the solid, beat it with water and centrifuge, take out the solid and add it to the kettle, add water to control the temperature, stir and wash, centrifuge, and spin dry to obtain the product carbacrol.
Main reference materials
[1] Sun Yaoran, Guo Zhongliang, Qing Yingying, Wang Shiyong, Bai Xiaojun. Research on the synthesis process of carbacrol intermediate chloroacetylcatechol [J]. Journal of Shijiazhuang University, 2017, 19(03): 16-21 .
[2] [Invented in China] CN201711193729.5 A production process of adrenal color hydrazone

 微信扫一扫打赏
微信扫一扫打赏

