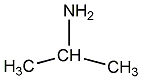
Structural formula
| Business number | 01JG |
|---|---|
| Molecular formula | C3H9N |
| Molecular weight | 59.11 |
| label |
2-Aminopropane, 1-Methylethylamine, Monoisopropylamine, Nitrogen-containing compound solvents, hard water treatment agent, Detergent |
Numbering system
CAS number:75-31-0
MDL number:MFCD00008082
EINECS number:200-860-9
RTECS number:NT8400000
BRN number:605259
PubChem number:24870715
Physical property data
1. Properties: Colorless and volatile liquid with a fishy ammonia odor. [1]
2. Melting point (℃): -101.2~-95[2]
3. Boiling point ( ℃): 33~34[3]
4. Relative density (water=1): 0.69[4]
5. Relative vapor density (air=1): 2.03[5]
6. Saturated vapor pressure (kPa): 77.27 (25℃)[6]
7. Heat of combustion (kJ/mol): -2345.5[7]
8. Critical temperature (℃): 198.6[8]
9. Critical pressure (MPa): 4.54[9]
10. Octanol/water partition coefficient :0.26[10]
11. Flash point (℃): -26 (OC)[11]
12 .Ignition temperature (℃): 402[12]
13. Explosion upper limit (%): 12[13]
14. Lower explosion limit (%): 2.3[14]
15. Solubility: miscible with water, miscible in ethanol and ether, easily soluble in acetone , soluble in benzene and chloroform. [15]
16. Viscosity (mPa·s, 25ºC): 0.36
17. Heat of evaporation (KJ/mol, 25ºC): 28.5
18. Heat of evaporation (KJ/mol, b.p.): 27.21
19. Heat of formation (KJ/kg, 25ºC, liquid): -112.33
20 . Heat of formation (KJ/kg, 25ºC, gas): -83.82
21. Heat of combustion (KJ/mol, 25ºC): 2356.08
22. Specific heat capacity (KJ/(kg ·K), 25ºC, constant pressure): 2.78
Toxicological data
1. Acute toxicity[16]
LD50: 111mg/kg (rat oral); 380mg/kg (rabbit dermal )
LC50: 4000ppm (rat inhalation, 4h)
2. Irritation [17]
Rabbit transdermal: 345 mg, moderate stimulation (open stimulation test).
Rabbit eye: 50μg, severe irritation.
Ecological data
1. Ecotoxicity[18] EC50: 91.5mg/L (48h) (Water flea)
2. Biological Degradability No information available
3. Non-biodegradability [19] In the air, when the hydroxyl radical concentration is 5.00× At 105 pieces/cm3, the degradation half-life is 10h (theoretical).
4. Other harmful effects [20] This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Mount��Refractive index: 19.44
2. Molar volume (cm3/mol): 82.1
3. Isotonic specific volume (90.2K): 177.9
4. Surface tension (dyne/cm): 22.0
5. Polarizability (10-24cm3) :7.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 10.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has the chemical properties of primary amines, and its aqueous solution is alkaline. Mixtures with air are highly explosive.
2. Stability[21] Stable
3. Incompatible substances[22] Acids, acid chlorides, acid anhydrides, strong oxidants, carbon dioxide
4. Polymerization hazards [23] No polymerization
5. Decomposition products[24] Ammonia
Storage method
1. This product is a first-class flammable liquid. Packed in iron drums. The container should be sealed and stored in a cool and ventilated place. Fireworks are strictly prohibited. It should be isolated from oxidizing agents during storage. In the event of a fire, use carbon dioxide, sand, dry chemical powder or mist water to extinguish the fire.
2. Storage precautions [25] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 29℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
There are two main methods for producing isopropylamine in industry:
1. Acetone hydrogenation and amination method. The raw material acetone is fed into a reactor with copper-nickel-clay clay as the catalyst. Under normal pressure At a temperature of 150-220°C, hydrogen and ammonia are introduced for reaction. The reaction product is purified by distillation to obtain monoisopropylamine, and diisopropylamine is also generated. The conversion rate of acetone reaches 98%, and the total yield of mono- and diisopropylamine reaches more than 90%. Raw material consumption quota: acetone 1390kg/t, liquid ammonia 450kg/t.
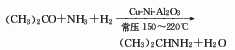
2. Isopropyl alcohol hydrogenation amination method Isopropyl alcohol reacts with ammonia and hydrogen at 195°C and 1.72MPa through a porous nickel-aluminum catalyst activated by barium hydroxide to generate monoisopropylamine and diisopropylamine. The total reaction conversion rate can reach 86%, and the product yield (based on isopropyl alcohol) reaches 96%, which contains 37% monoisopropylamine, 33% diisopropylamine, 12% isopropyl alcohol, and 18% water.
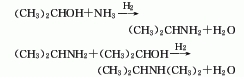
Refining method: Isopropylamine is used It is produced by reacting isopropyl bromide with an alcoholic solution of ammonia, or by reacting acetone with ammonia and hydrogen under the catalysis of nickel-copper-clay clay. Therefore, it may contain impurities such as acetone, isopropyl bromide, isopropyl alcohol and various other propylamines. It can be refined by distillation. To obtain high-purity isopropylamine, add anhydrous barium oxide and let it sit for several days, then distill it in the presence of sodium, collect the fraction at 31.4°C under 131.961 Pa, and distill it again.
Purpose
1. Used as synthetic pesticides, medicines, dye intermediates, rubber vulcanization accelerators, emulsifiers, detergents, detergents, depilators, hard water treatment agents, surfactants and textile auxiliaries, etc. Produces the pesticide badan and the herbicides atrazine, atrazine, permethrin, fenacetate and other herbicides, which are used medicinally for Ganle, indanol hydrochloride, propranolol hydrochloride, and indololol Waiting for drugs. Used as solvent, hard water treatment agent, detergent.
2. Used as solvents, intermediates in organic synthesis, emulsifiers, surfactants, and rubber vulcanization accelerators. [26]

 微信扫一扫打赏
微信扫一扫打赏

