Background and overview[1]
1-Chloro-4-(4-phenyl-1,3-butadiene)benzene can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
1) Synthesis of bisethyl-4-chlorobenzylphosphine
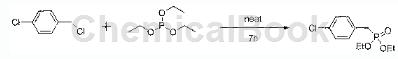
Preparation process: Preparation of bisethyl-4-chlorobenzylphosphine: 1-chloro-4-(chloromethyl)benzene (50g, 0.31mol) and triethyl phosphite (74.9g, 0.47mol) Add to a 200 ml flask, heat to reflux and stir for 7 hours. The mixture is cooled to room temperature, and excess triethyl phosphite is distilled off under reduced pressure to obtain a crude product. The product in this step does not need to be purified and can be directly used in the next step of reaction.
2) Synthesis of 41-chloro-4-(4-phenyl-1,3-butadiene)benzene
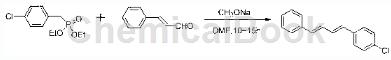
Preparation process: Add the product in 1 (39.4g, 0.15mol) to 100ml dry DMF, add 28% sodium methoxide (28.9g, 0.15mol) to the above solution, stir evenly, and add cinnamaldehyde (15.8g, 0.12mol)/DMF (10ml) solution was added to the mixture. After stirring at room temperature for 10~15h, the mixture was poured into 300ml of water, and a small amount of HCl solution was added to obtain a large amount of white solid. Filter it and use 20ml of methanol. washes. Then pour the white solid into 100ml methanol, stir, filter (twice), and dry under reduced pressure at 70°C to obtain white powder 1-chloro-4-(4-phenyl-1,3-butadiene)benzene , about 26g, yield 90%. (Melting point, characterization data).
Application
1-Chloro-4-(4-phenyl-1,3-butadiene)benzene can be used as a pharmaceutical synthesis intermediate, if the following reaction occurs:
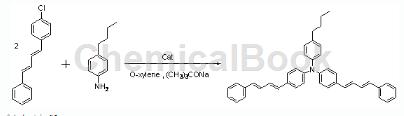
Preparation process: Accurately weigh 5.70g (0.02mol) of the product synthesized in the second step, 1-chloro-4-(4-phenyl-1,3-butadiene)benzene, in the glove box, 0.035g ( 0.1 mmol) 2-(dicyclohexylphosphino)biphenyl, 2.9 g (0.03 mol) sodium tert-butoxide and 0.022 g (0.1 mmol) palladium acetate. Under Ar gas protection, 1.49g (0.01mol) p-n-butylaniline and 150ml anhydrous and oxygen-free o-xylene were injected into the mixture, and the mixture was reacted at 130°C for 5 hours. After the reaction is completed, distill under reduced pressure at 80°C to obtain a brown liquid. Cool to 15°C overnight to obtain a brown solid; after washing with o-xylene, dissolve the solid in o-xylene at 70°C. , add silica gel, stir for 2 hours, filter while hot, and wash the silica gel with o-xylene. Collect the filtrate, distill under reduced pressure at 80°C, and cool to 15°C to obtain a yellow solid; filter, then wash with o-xylene, and finally use Wash with ethyl acetate and dry under reduced pressure at 70°C to obtain yellow-green powder. Yield 66%. Characterization: m.p.160~161°C.1HNMR (400MHz, CDCl3): 7.42 (d, 4H, J=8Hz), 7.29 (d, 8H, J=12Hz), 7.21 (d, 2H, J=8Hz), 7.08 (d, 2H, J=8Hz), 7.02 (d, 6H, J=8Hz), 6.97-6.91 (m, 2H), 6.87-6.81 (m, 2H), 6.62 (d, 2H, J=4Hz), 6.60 (d, 2H, J=4Hz), 2.58 (t, 2H, J=8Hz), 1.64-1.56 (m, 2H), 1.42-1.33 (m, 2H), 0.94 (t, 3H, J=8Hz) .
Main reference materials
[1] (CN102516088) Synthesis of a triphenylamine compound with strong fluorescence

 微信扫一扫打赏
微信扫一扫打赏

