Background and overview[1]
Phenol is commonly known as carbolic acid. The simplified structural formula is C6H5OH. Colorless or white crystals that turn pink in air or sunlight. Slightly soluble in water, easily soluble in ethanol, ether, chloroform, glycerin, carbon disulfide, etc., almost insoluble in petroleum ether and paraffins. It is widely used as raw material or solvent in the manufacturing of dyes, synthetic resins, plastics, synthetic fibers and pesticides. Acute toxicity: Oral LD50 for mice is 300mg/kg, and for rats is 530mg/kg. The amount of phenol in urine of people at the production site is highly correlated with the exposure, so the amount of phenol in urine can be used as an indicator of exposure to phenol. Strongly corrosive to skin and mucous membranes. The absorption rate of inhaled vapor by respiratory organs is very high. The symptoms of acute poisoning caused by vapor absorption include headache, dizziness, vomiting, tinnitus, insomnia, etc. In addition to central nervous system symptoms, chronic poisoning can also cause cough, loss of appetite, diarrhea, and liver and kidney disorders. The oral lethal dose is 15g or 1.5g.

The maximum allowable concentration of phenol in the working environment air is 5ppm (19mg/m3, skin absorption) in the United States, the Federal Republic of Germany and Japan, 5mg/m3 (skin absorption) in China and the Soviet Union, and 20mg in Czechoslovakia and the United Kingdom. /m3 and 19mg/m3 (5ppm, time-weighted average), 40mg/m3 and 38mg/m3 (upper limit) (applicable to phenol and its compounds); in the atmosphere, the Democratic Republic of Germany, Yugoslavia, and Bulgaria are all stipulated as 0.0026ppm ( 0.01mg/m3), Czechoslovakia is 0.01mg/m3 (applicable to phenol and its compounds); Czechoslovakia stipulates 0.05mg/L in drinking water, and the World Health Organization (WHO) recommends 0.02mg/L (the highest expected concentration is 0.001mg/L) (applicable to all phenols), and the European Economic Community (EEC) stipulates that it is 0.5μg/L. Japanese wastewater discharge standards stipulate that total phenols are 5mg/L.
Function and use[1]
Phenol is a disinfectant and antiseptic. Phenol drugs are all protoplasmic poisons, which can coagulate and precipitate proteins to exert antibacterial effects and have strong tissue penetration. It has a bacteriostatic effect at low concentrations (0.0125% ~ 0.02%), and has a killing effect on bacteria and fungi at high concentrations (1% ~ 2%), but is ineffective against bacterial spores and viruses. It can fully function under acidic conditions, but is less effective under alkaline conditions or in the presence of organic matter. Low concentrations have irritating and local anesthetic effects on skin and mucous membranes, while high concentrations have corrosive effects. The aqueous solution has the strongest antibacterial effect, while the antibacterial effect and irritation of oil and glycerol solutions are weakened. It is often used clinically for the disinfection of instruments, utensils and houses, but it is corrosive to metal instruments. Also used for skin itching.
Usage and dosage[1]
For disinfection: 3% ~ 5% aqueous solution.
For skin itching: 0.5% to 1% solution or lotion.
For acute otitis media, otitis externa and acute myringitis: 1% to 3% glycerol solution.
Adverse reactions[1]
Phenol is corrosive to skin and mucous membranes and can cause burns on contact. The concentration of aqueous solutions used on the body surface exceeds 2%, which can cause tissue damage or even necrosis.
Notes[1]
1. If burns occur, wash immediately with vegetable oil or 50% ethanol. If the wound is large, rinse it with plenty of water and then apply oil on it.
2. This product can be toxic if absorbed through the skin and mucous membranes, so care should be taken when using it.
3. Rubber, plastic and fiber products can absorb phenol, so they cannot be disinfected with phenol. Skin contact with the above disinfection items can cause burns.
4. This product is highly toxic and is now used sparingly.
Preparation[2]
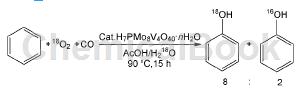
Direct oxidation of benzene with oxygen to produce phenol: In the gas phase benzene oxidation reaction, reducing gases such as H2, NH3 and CO are widely used as reducing agents; in the liquid phase benzene oxidation reaction, there are also research reports on reducing gases as reducing agents. . Using Pd/Pt/SAC13 as the catalyst, hydrogen as the reducing agent, and the reaction gas is H2:O2:N2=1:1:1, phenol can be obtained. Adding NaBr additive to the reaction helps to increase the yield of phenol, and it is pointed out that this is because the bromide ions in NaBr can promote the generation of hydrogen peroxide on the palladium-containing catalyst. CO can be used as a reducing agent. In the reaction, CO reduces the H7PMo8V4O40 catalyst to H7PMo8V4O40-x, and oxygen activates the vanadium species of the partially reduced H7PMo8V4O40-x catalyst. The isotope tracking reaction through 16O2 and H182O shows that there is no in-situ generation of hydrogen peroxide intermediate product (H16O18OH) during the reaction, but oxygen is activated on the vanadium species in the partially reduced H7PMo8V40-x catalyst, and the activated oxygen directly interacts with Benzene reacts to form phenol.
Other uses[3-4]
Phenol can be used as an intermediate in pharmaceutical synthesis. Examples of its applications are as follows:
1) Preparation of diphenyl carbonate. This method uses phenol and dimethyl carbonate as raw materials and carbon nanotube-supported titanium dioxide as a catalyst. Carbon nanotube-supported titanium dioxide as a catalyst can be prepared by sol-gel method, impregnation method, or hydrothermal method. The catalyst uses carbon nanotubes CNT as a carrier and titanium dioxide as an active component. The catalyst used in the method of the invention has high activity for reacting dimethyl carbonate and phenol to prepare diphenyl carbonate, good selectivity and high stability, and can make the total yield of diphenyl carbonate and alkylphenyl carbonate reach 49.0%. , no by-products were generated, and after the catalyst was reused four times, the phenol conversion rate remained above 45%. Carbon nanotube supported titanium dioxide catalystThe catalyst is a heterogeneous catalyst that is easy to separate, recyclable and has potential industrial application value.
2) An industrial-scale continuous method for producing and recovering cyclohexanone from phenol and hydrogen, the method comprising: hydrogenating phenol in a phenol hydrogenation reactor; in a separation and purification section containing at least 4 distillation sections [II] wherein cyclohexanone is separated from the hydrogenation product stream; wherein at least a part of the reaction heat generated in the phenol hydrogenation reaction section [I] is used to produce steam; and wherein the cyclohexanone and the hydrogenation product stream added to the phenol hydrogenation reactor are The molar ratio of phenol is 0.02 to 0.10; and/or the molar ratio of cyclohexanol and phenol added to the phenol hydrogenation reactor is 0.001 to 0.10.
Main reference materials
[1]Practical Drug Handbook
[2] Direct oxidation of benzene with oxygen to prepare phenol
[3] CN201010263740.6 A method for synthesizing diphenyl carbonate by transesterification of dimethyl carbonate
[4] CN201580060894.6 Method for producing cyclohexanone from phenol

 微信扫一扫打赏
微信扫一扫打赏

