Overview[1]
P-methylanisole is a colorless and transparent liquid with a strong floral aroma. It is prepared by reacting p-cresol with dimethyl sulfate in an alkaline solution. It can be used to prepare artificial orchids, narcissus and other flavors. At present, the method of synthesizing p-methyl anisole mainly involves the reaction of p-cresol and methylating reagents, such as dimethyl carbonate (DMC), methyl iodide, dimethyl sulfate (DMS), etc. Among them, methyl iodide is Due to its high price and strict operating requirements, it is not widely used. DMC is being widely studied as an emerging industrial reagent.
Apply[2]
P-methylanisole (p-methylanisole) is an anisole derivative that naturally exists in ylang ylang oil and cananga ylang oil. It has a violet aroma and a ylang ylang floral aroma. It is a very important chemical raw material and is widely used in the fields of medicine, spices and organic synthesis intermediates. For example, in the field of spices, it is widely used to prepare various flavors such as walnuts and hazelnuts. It is also used as an organic synthesis intermediate to synthesize a series of chemical products such as anisaldehyde.
Preparation[2-3]
Method 1: Use tetrabutylammonium bromide as a phase transfer catalyst to catalyze the reaction of p-cresol and dimethyl sulfate to synthesize p-methyl anisole. The results show that in the mole ratio of dimethyl sulfate and p-cresol The yield of p-methyl anisole can reach up to 92.36% when the ratio is 1.4, the amount of phase transfer catalyst is 6%, pH=8, the water bath temperature is controlled at 50-60°C, and the reaction time is 6 hours.

Add 10.5 mL (0.1 mol) of p-cresol and 30 mL of toluene into a three-necked flask equipped with a magnet, thermometer, dropping funnel, and spherical reflux condenser. Stir magnetically while heating the water bath to 50°C. Add to the reaction flask Add 1.93g (0.006mol) of phase transfer catalyst tetrabutylammonium bromide, use a certain amount of 30% K2CO3 solution to adjust the pH to alkaline, control the temperature at 50-60°C, continue stirring for 40min, and slowly drip ( CH3)2SO4 solution 13.23mL (0.14mol), the dropping time is 50min, and the pH of the solution is controlled at the same time = 8. After the dripping is completed, the reaction is refluxed at the same temperature for 6h. When the reaction is completed, the upper organic layer is washed with 3×20mL 10% ammonia water, and the water layer is separated and removed. , the organic layer was washed with saturated NaCl solution until neutral, dried with anhydrous CaCl2, filtered, and distilled under reduced pressure to collect the product. The product yield was 92.36%.
Method 2: A method for preparing p-methylanisole, specifically including the following steps:
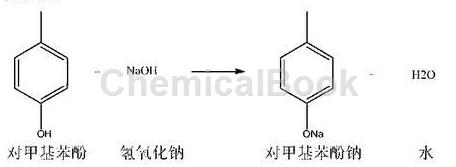
1) Neutralization reaction: 100.0g of p-methylphenol put into a 1000ml four-necked flask equipped with a thermometer, stirring paddle, nitrogen pipe, and recovery condenser (content: 99.1%, 0.9176mol), none Water ethanol: 600ml, turn on nitrogen protection, set nitrogen flow rate: 0.5l/min. Cool the outer wall of the four-necked flask with cooling water, and control the internal temperature not to exceed 35°C. Slowly add 37.5g (0.9375mol) of sodium hydroxide from the thermometer port (pull out the thermometer) into the four-necked flask. After the feeding is completed, stir and react for 1.0 hours. After the reaction is completed, the outer wall of the four-necked flask is heated with 85-90°C hot water, and the ethanol is continuously steamed out under nitrogen protection. In the later stages of distillation, the outflow velocity of ethanol will become smaller and smaller. When the ethanol outflow is extremely small, vacuum recovery is performed, and the vacuum degree is controlled to be greater than -0.08MPa to recover the ethanol to dryness. The residue in the four-necked flask is sodium p-methylphenolate, quantity: 121.1g, content: approximately 98.2%.
2) Methylation reaction 1: Put the prepared sodium p-methylphenolate: 50.0g (content: about 98.2%, 0.3777mol) into a 500ml four-necked flask equipped with a thermometer, stirring paddle and reflux condenser. , Cyclohexane: 300ml. The outer wall of the four-necked flask is insulated with 40°C warm water, start stirring, and adjust the stirring speed to be greater than 500r/min. Weigh dimethyl sulfate: 50.0g (content: 98.7%, 0.3917mol) and add it to the constant pressure dropping funnel, and then add the dimethyl sulfate in the constant pressure dropping funnel dropwise to the fourth volume within 30 to 60 minutes. Carry out methylation reaction in a mouth flask. After the dropwise addition of dimethyl sulfate is completed, keep it warm for 5.0 to 8.0 hours.
3) Post-processing: After heat preservation, filter after cooling. The filter cake was washed with 100 ml of cyclohexane and dried to obtain sodium methyl sulfate: 50.8g, content: 97.3%. The filtrate is washed with alkaline water and the solvent is recovered (the washed alkaline water is used again). After the solvent recovery is completed, p-methylanisole is obtained: 45.6g, content: 98.1%, yield: 97.0%.
4) Methylation reaction 2: In a 500ml three-necked flask equipped with a thermometer, stirring paddle, and reflux condenser, add sodium p-methylphenolate: 42.5g (content: about 98.2%, 0.3210mol), methyltetrahydrofuran : 300ml, dried sodium methyl sulfate: 50.8g (content: 97.3%, 0.3689mol). Turn on stirring, heat the outer wall of the three-necked flask with an electric heating mantle, and conduct reflux reaction for 5.0 to 8.0 hours. After the reaction is completed, cool to room temperature.
5) Post-processing: After cooling, filter, and the filter cake is sodium sulfate for comprehensive utilization. The filtrate was transferred to a 500ml three-necked flask to recover methyltetrahydrofuran. After recovery, p-methylanisole was obtained: 38.5g, detected content: 97.2%, yield: 95.5%.
Main reference materials
[1] Dictionary of Chemical Substances
[2] Phase transfer catalytic synthesis of p-methylanisole
[3] CN201910295168.2 Preparation method of p-methyl anisole

 微信扫一扫打赏
微信扫一扫打赏

