Overview[1]
Since phosphorus has an outer electron configuration of 3S23P3 and is a Lewis base, its organophosphine compounds can serve as good electron donors in coordination chemistry, forming feedback bonds with metal atoms to obtain stable coordination compounds. Organophosphines Due to their unique properties, ligands have always been a hot research topic in the field of catalysis, especially in the field of asymmetric catalysis, and have entered industrial production. Tris(p-tolyl)phosphine has a wide range of uses, such as as a catalyst ligand for coupling reactions; in terms of materials, it is used in the synthesis of flame retardants; in terms of organic synthesis, it is an important organic intermediate.
Preparation[1]
There have been many related reports on its preparation. The literature uses tris(p-tolyl)phosphine as the raw material reduction method. Although the yield of the reduction method is relatively high, up to 90%, the raw material cost is high. It is more expensive than the product itself, and acetonitrile is used as the solvent during the reaction, which is highly toxic; it is produced by a pro-reaction using p-methylphenyllithium and phosphorus trichloride as raw materials. This method uses p-methylphenyllithium as the raw material. However, the preparation conditions of lithium reagent are harsh and the price is more expensive than Grignard reagent. It uses p-bromotoluene as raw material and is prepared through Grignard reaction and nucleophilic reaction. However, the price of p-bromotoluene is 8 times that of p-chlorotoluene used in this study. , the molecular weight is 1.36 times that of p-chlorotoluene, the overall raw material cost is about 11 times that of p-chlorotoluene, and using bromine as raw material has more Grignard coupling by-products; directly use p-methylphenyl magnesium chloride and phosphorus trichloride It is prepared by the nucleophilic reaction. This method uses the catalyst (Cl4Cu) 2-2Li+ during the reaction process, which is relatively expensive. Moreover, p-chlorotoluene is the raw material of methylphenyl magnesium chloride. Using p-chlorotoluene as the raw material can save more costs. The preparation conditions for Grignard reagents are harsh, and the impact of water is only important for small reactions (especially mmol level). Because they are all prepared under nitrogen protection conditions and reflux conditions, the impact of water on 1L reactions It’s relatively small.
The synthesis route of tris(p-tolyl)phosphine is divided into two steps: Step (1), preparation of Grignard reagent. Since the initiation of Grignard reagent for p-chlorotoluene is difficult, Grignard reagent is the best In this article, the Grignard reagent of p-bromotoluene is used as the initiator (p-bromotoluene and magnesium chips are used as raw materials, tetrahydrofuran is used as the solvent, iodine is used to initiate the reaction, and about p-methylphenyl magnesium bromide is synthesized as the initiator. Initiate the synthesis of p-methylphenyl magnesium chloride from p-chlorotoluene). The synthesis route of the initiator p-methylphenyl magnesium bromide is shown in formula (1). Then use p-methylphenyl magnesium bromide as the initiator, and use p-chlorotoluene , magnesium chips as raw materials, tetrahydrofuran as solvent, prepare Grignard reagent p-methylphenylmagnesium chloride, the reaction route is shown in formula (2). Step (2), preparation of tris(p-tolyl)phosphine. Put step (1) The nucleophilic reaction between p-methylphenylmagnesium chloride prepared in the step and phosphorus trichloride is used to prepare tris(p-tolyl)phosphine. The synthesis route is shown in formula (3).
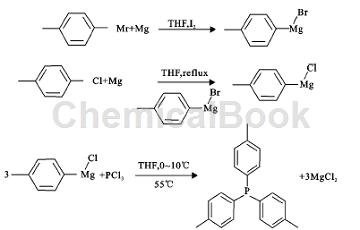
1) Preparation of Grignard reagent
Prepare 1 mixed solution of p-chlorotoluene and THF (or recycled THF). Under nitrogen protection, add 20 ml of p-chlorotoluene and THF mixed solution and magnesium chips into the flask, and heat the flask until the solvent refluxes. Under reflux conditions , add the previously prepared Grignard reagent p-methylphenylmagnesium bromide to initiate the reaction. After the initiation is successful, add the remaining mixed solution of p-chlorotoluene and THF dropwise. After the dropwise addition is completed, incubate under reflux conditions until The reaction of the raw materials is complete, and the Grignard reagent p-methylphenylmagnesium chloride is prepared. Pretreatment for reaction detection: take the reaction solution and slowly add saturated ammonium chloride aqueous solution to quench the reaction. Since p-methylphenylmagnesium chloride will hydrolyze toluene, it can Use thin layer chromatography to check whether the raw material p-chlorotoluene has reacted completely. Because the polarity of toluene and p-chlorotoluene is relatively small, petroleum ether can be used as a developing agent to separate the two. At the same time, because the molecule has a conjugated structure such as a benzene ring , you can choose UV color development method.
2) Preparation of tris(p-tolyl)phosphine
After cooling down the Grignard reagent (p-methylphenyl magnesium chloride), pump it into a 1L four-necked flask. At different internal temperatures, add dropwise the mixed solution of PCl3 and THF (volume ratio 1:1) to ��In a four-necked flask with Grignard reagent p-methylphenylmagnesium chloride (control the same temperature and add the Grignard reagent dropwise to the mixed solution of phosphorus trichloride and tetrahydrofuran, and examine the effect of the back-drip addition on the reaction), Then heat under reflux conditions for 2 hours until the reaction of the Grignard reagent with p-tolylmagnesium chloride is complete, and the target product tris(p-tolyl)phosphine is obtained.
3) Post-treatment of tris(p-tolyl)phosphine
After distilling out the solvent tetrahydrofuran in the reaction, add a certain amount of water and methylene chloride to separate the solution system into two phases (aqueous phase and organic phase). Keep still to clarify both phases, and then separate the water phase. come out; distill out dichloromethane under normal pressure until solid matter precipitates; cool down, recrystallize the solid matter with methanol, and finally obtain the pure target product tris(p-tolyl)phosphine.
Main reference materials
[1] Synthesis and characterization of tris(p-methylphenyl)phosphine

 微信扫一扫打赏
微信扫一扫打赏

