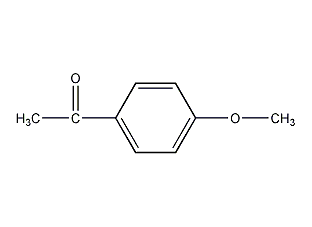
Structural formula
| Business number | 02H2 |
|---|---|
| Molecular formula | C9H10O2 |
| Molecular weight | 150.17 |
| label |
4′-Methoxyacetophenone, 4-methoxyacetophenone, Acetyl anisole, Hawthorn flower ketone, P-Acetanisole, 4-Methoxyphenylethanone, 4′-Methoxyacetophenone, p-Methoxyphenyl methylketone |
Numbering system
CAS number:100-06-1
MDL number:MFCD00008745
EINECS number:202-815-9
RTECS number:AM9240000
BRN number:742313
PubChem number:24883507
Physical property data
1. Characteristics: Colorless to slightly yellow crystal blocks, with a strong and lasting hay aroma, a somnocin-like aroma, and floral and hawthorn aromas.
2. Relative density (g/mL, 41.1℃): 1.0818
3. Relative density (20℃, 4℃): 1.081841
4. Melting point (ºC): 38~39
5. Boiling point (ºC, normal pressure): 258
6. Boiling point (ºC, 3.5kpa) :152~154
7. Boiling point (ºC, 1.33kpa): 136
8. Flash point (ºC): 138
9. Refractive index(n41D): 1.547
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of the (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Insoluble in water, soluble in alcohol and ether.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24HREACTION SEVERITY, moderate reaction; 2. Acute toxicity: human inhalation TCL0: 1700μg/m3/39W-I; Rat oral LD50: 1720mg /kg; Mouse oral LD50: 820mg/kg; Rabbit skin contact LD50: >5mg/kg; 3. Other multiple dose toxicity: Rat inhalation TCLo: 152mg/m3/4H/13W-I;
EcologyData
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 42.95
2. Molar volume (cm3/mol): 144.9
3. Isotonic specific volume (90.2K ): 349.1
4. Surface tension (dyne/cm): 33.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with light and oxides.
2. Found in flue-cured tobacco leaves.
3. Naturally found in beef, sour fruits, and guava.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Store away from light and tightly packed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It is prepared by acetylation reaction between anisole and acetic anhydride (or acetyl chloride) under the conditions of anhydrous aluminum trichloride (or ferric chloride, zinc chloride, acidic clay). Add anisole and acetic anhydride to the reaction pot, add zinc chloride and slowly heat to boiling, maintain reflux (about 140°C), react for 4 hours, distill under reduced pressure, wash with water, and rectify to obtain the finished product.
2. Obtained from the reaction of anisole and acetic acid in the presence of boron trifluoride.
3. Obtained from the reaction of anisole and acetyl chloride in the presence of aluminum chloride and carbon disulfide.
4. Tobacco: FC, 18.
Purpose
1. It has a strong hawthorn aroma and is an indispensable raw material for fragrances such as mimosa and hawthorn. It is often used in high-end cosmetics and soap flavors. It has high stability in soap and can also be used as fruit food flavor. . It is also used in organic synthesis to produce p-methoxyphenylacetic acid and is used as an intermediate for puerarin. The dosage in the fragrance formula is within 5%. IFRA has no restrictions. It can be used in edible and tobacco flavor formulations.
2. Used in baked goods, meat products, and candies.

 微信扫一扫打赏
微信扫一扫打赏

