Background and overview[1][4]
N-Phenyl anthranilic acid is a sensitive internal oxidation-reduction indicator. It is especially suitable for the determination of vanadium in steel analysis. Also known as vanadium reagent, it is an important intermediate commonly used in the synthesis of acridine compounds and is widely used in antimalarial drugs, anti-inflammatory drugs, anti-tumor drugs, etc. Molecular formula C13H11NO2. Molecular weight 213.24. Leaf-like crystals. Melting point 183 ~ 184℃ (decomposition). Soluble in hot ethanol, insoluble in hot water, hot benzene and ether.
Preparation[4]
Add solvent into the reaction flask with a thermometer and reflux tube, add a certain amount of ortho-halobenzoic acid, potassium carbonate, catalyst and aniline in sequence while stirring, heat the system and then reflux to start the reaction. TLC tracking shows that the reaction is complete. Then stop heating. Add cooling water to cool the system to 60-80°C, add activated carbon and sodium sulfide nonahydrate, continue stirring until the system is uniform and then filter, then add concentrated hydrochloric acid to adjust the pH of the filtrate to 1-2, filter, wash and dry the precipitate. After purification by column chromatography, N-phenyl anthranilic acid is obtained. The reaction equation is as follows:
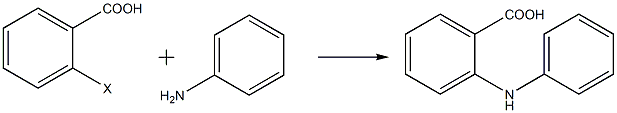
Hu Xiaolun and others used o-halobenzoic acid and aniline as the main raw materials and synthesized N-phenyl anthranilic acid using the Ullmann condensation method. They studied the effects of raw materials, catalysts, temperature, solvent and other factors on the reaction. Experimental results show that o-iodobenzoic acid has higher reactivity; the activity of iron-copper oxide catalyst is not sensitive to temperature changes and has good catalytic effect at different temperatures, while copper powder, copper oxide or copper chloride is used as the catalyst. When used as a catalyst, the higher the reaction temperature, the better the catalytic effect; when dimethyl sulfoxide (DMSO) is used as the solvent, the overall product yield is higher, up to 92%.
Apply[2-3] [5]
CN201410437363.1 discloses a method for determining vanadium element in ferrovanadium alloy. This method uses potassium permanganate to oxidize vanadium into pentavalent in a sulfur-phosphorus mixed acid medium. Excess potassium permanganate is used in the presence of urea. For sodium nitrite reduction, use N-phenyl anthranilic acid as an indicator and titrate with ferrous ammonium sulfate standard solution. The measurement range is above 0.5%. This method reduces the weighing amount to 0.1g, and changes the sample dissolving process to: add 20-50mL of hydrochloric acid, 5-15mL of nitric acid, and 10mL of phosphoric acid, heat slightly until the sample is dissolved, add 30mL of 1+1 sulfuric acid, and heat to evaporate. Emit sulfuric acid fumes for 2-3 minutes. Cool slightly, add 50mL of water, and heat to dissolve the salts. After this improvement, it is easy to completely dissolve the sample, and the sample dissolution time is greatly shortened, which improves work efficiency without affecting the accuracy of the test results.
CN201410122824.6 discloses a method for measuring the cerium sulfate content of tin-cerium alloy electroplating solutions. It belongs to the technical field of electroplating solution analysis. Its main technical feature is that it uses a certain concentration of perchloric acid, phosphoric acid, and nitric acid to test the electroplating solution. Carry out oxidation treatment, use N-phenyl anthranilic acid as an indicator, and use ferrous ammonium sulfate standard titration solution to titrate tetravalent cerium into trivalent cerium to determine the cerium sulfate content in the tin-cerium alloy electroplating solution. This determination The method has the characteristics of simple detection, convenient, fast and environmentally friendly detection process, low cost and accurate and consistent detection results.
CN201410415911 discloses a method for measuring the concentration of tetravalent and pentavalent vanadium ions in the vanadium battery electrolyte. The method is to use potassium permanganate to oxidize tetravalent vanadium into pentavalent vanadium in the positive electrode test solution under sulfuric acid medium. In the presence of urea, use sodium nitrite to decompose excess potassium permanganate, use N-phenyl anthranilic acid as an indicator, and titrate with ferrous ammonium sulfate standard solution to determine the total vanadium ion concentration in the electrolyte. In another positive electrode test solution, under sulfuric acid medium, use N-phenyl anthranilic acid as an indicator and titrate with ferrous ammonium sulfate standard solution to measure the concentration of pentavalent vanadium ions in the positive electrode electrolyte. The total vanadium ion concentration minus the pentavalent vanadium ion concentration is the tetravalent vanadium ion concentration. The method is not only easy to operate, but also has high accuracy in measuring the concentration of tetravalent and pentavalent vanadium ions in the vanadium battery electrolyte. Its measurement results have good stability, reproducibility and accuracy, and can fully satisfy the daily measurement of vanadium battery electrolysis. The concentration of tetravalent and pentavalent vanadium ions in the liquid is required.
Main reference materials
[1] Chemical Dictionary
[2] CN201410437363.1 A method for determining vanadium element in ferrovanadium alloy
[3] CN201410122824.6 A method for measuring the cerium sulfate content of tin-cerium alloy electroplating solution
[4] Hu Xiaolun, Zhao Pingping, Yang Guangyuan, Hao Jiajin. Research on the synthesis of N-phenyl anthranilic acid by Ullmann condensation method [J]. Progress in Fine Petrochemicals, 2016, 17(03): 56-58.
[5] CN201410415911.0 A method for measuring the concentration of tetravalent and pentavalent vanadium ions in vanadium battery electrolyte

 微信扫一扫打赏
微信扫一扫打赏

