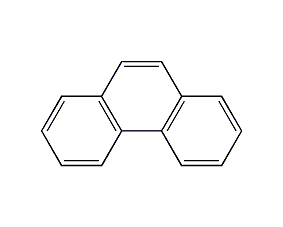
Structural formula
| Business number | 01VB |
|---|---|
| Molecular formula | C14H10 |
| Molecular weight | 178.23 |
| label |
pulp anti-fogging agent, stabilizer, hydrocarbon solvents, dye synthesis intermediates, aromatic compounds |
Numbering system
CAS number:85-01-8
MDL number:MFCD00001168
EINECS number:201-581-5
RTECS number:SF7175000
BRN number:1905428
PubChem number:24855775
Physical property data
1. Character: white shiny and fluorescent flaky crystals
2. Relative density (d44) :0.983. Relative density (25℃, 4℃): 1.0581004. Melting point (ºC): 100
5. Boiling point (ºC, normal pressure): 340
6. Boiling point (ºC, 1.6kPa): 210-215℃ 7. Refractive index (n20D): 1.59438. Crystalline phase standard combustion Heat (enthalpy) (kJ·mol-1): -7054.48
9. The crystal phase standard claims heat (enthalpy) (kJ·mol-1): 116.19
10. Autoignition point or ignition temperature (ºC): 18.5
11. Solubility parameter (J·cm-3)0.5 : 20.067
12. van der Waals area (cm2·mol-1): 1.084×1010
13. van der Waals volume (cm3·mol-1): 99.560
14. Critical Temperature (K): 595.85
15. Crystal phase standard entropy (J·mol-1·K-1): 215.06
16. The standard free energy of formation of the crystal phase (kJ·mol-1): 270.68
17. The standard hot melt of the crystal phase (J·mol-1 ·K-1): 220.62
18. Lower explosion limit (%, V/V): 5.04
19. Solubility: Insoluble in water, slightly soluble in ethanol, soluble in ether, glacial acetic acid, benzene, carbon tetrachloride and carbon disulfide, etc.
20. Gas phase standard combustion heat (enthalpy) (kJ·mol– 1): -7145.8
21. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 207.5
22. Gas phase standard entropy (J·mol-1·K-1): 395.86
23. Gas phase standard formation free energy (kJ·mol-1): 308.2
24. Gas phase standard hot melt (J·mol-1·K-1): 185.7
Toxicological data
1. Acute toxicity:
Rat caliber LD50: 700mg/kg;
Mouse abdominal cavity LD50:700mg/kg; mouse intravenous LD50: 56mg/kg
2. Other multiple dose toxicity:
Mouse caliber TDL0: 6370 mg/kg/13W-I;
3. Chronic toxicity/carcinogenicity:
Mouse skin TDL0: 71 mg/kg; Mouse skin TDL0: 22 gm/kg/10W-I4. Harmful when taken orally, and harmful to eyes, respiratory system and skin Irritating. May cause cancer.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 61.93
2. Molar volume (cm3/mol): 157.6
3. Isotonic specific volume (90.2K): 414.9
4. Surface tension (dyne/cm): 47.9
5. Dielectric constant (F/m): 3.08
6. Polarizability (10 -24cm3): 24.55
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 4.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Topological molecular polar surface area (TPSA): 0
6. Number of heavy atoms: 14
7. Surface charge: 0
8. Complexity: 174
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. The number of uncertain atomic stereocenters: 0
12. The number of determined chemical bond stereocenters: 0
13. Uncertain chemical bond stereocenters Number of structural centers: 0
14. Number of covalent bond units: 1
Properties and stability
1. White, shiny and fluorescent flaky crystals that can sublimate in vacuum. Mixing dust and air can form an explosive mixture, with an explosion limit of 5.04g/m3 (lower limit).
2.Mice were given LD50700mg/kg orally and LD5056mg/kg intravenously. This product may cause cancer. Operators should wear rubber gloves, masks, work clothes, forced ventilation, and keep the concentration in the air below the explosion limit.
3. Exist in oriental tobacco leaves and smoke.
4. Sublimation in vacuum.
5. Can cause photosensitization to the skin.
Storage method
1. This product should be sealed and stored in a cool, dry place.
2. This product is packed into a solid wooden box, wooden barrel or plastic barrel, lined with plastic bags or kraft paper. The packaging opening should be tight and leak-proof, and the outside of the box should be tied tightly with iron wire and iron sheets. The net weight of each box shall not exceed 50kg. Store in open area.
Synthesis method
1. Phenanthrene is the most abundant component in coal tar, accounting for about 5% of coal tar, second only to naphthalene content. Anthracene oil in the 300-360°C distillation range of coal tar contains the most phenanthrene, followed by anthracene and carbazole. The extraction method of phenanthrene is usually to cool and crystallize anthracene oil, and then use vacuum filtration or centrifugal filtration to separate and remove the oil. There is a lot of phenanthrene dissolved in the oil, which can be recovered by precision distillation. The resulting crystal is called crude anthracene, which contains about 25-30% anthracene, 22-25% carbazole, and 30% phenanthrene. The crude anthracene is extracted with heavy benzene, cooled, and filtered. The filtrate is evaporated to remove the solvent and then recrystallized and filtered. The filtrate is distilled to obtain industrial phenanthrene, and refined phenanthrene can be obtained through sulfonation and crystallization.
2. Tobacco: OR, 57. Synthesis: Extraction with pentane.
3. It can also be synthesized with o-nitrobenzaldehyde and phenylacetic acid.
Purpose
1. Phenanthrene is oxidized to obtain phenanthrenequinone, which is used to replace the organic mercury pesticides Cialis and Sailisan. The biphenyl acid obtained by oxidation can be used to prepare alkyd resin. The oxidation of phenanthrene can also obtain phthalic anhydride, Cyclohexanone, phenol. Phenanthrene chlorinated products are used to make non-flammable electrical insulators and impregnants. The phenanthrenesulfonic acid obtained by phenanthrene sulfonation can be used to make binders, tanning materials, etc. But in fact, most of these application areas have yet to be developed. In the papermaking industry, phenanthrene can be used as an anti-fog agent for paper pulp; it can also be used in nitroglycerin explosives and nitrocellulose stabilizers and in the manufacture of smoke bombs; the solid oxide of phenanthrene can be used to make electrical insulation materials and fillers with good flame resistance. In medicine, phenanthrene can synthesize alkaloids – morphine and caffeine, dimethylmorphine and many other medicines with special physiological effects on reproductive organs. In the dye industry, phenanthrene can be used to produce 2-aminophenanthrenequinone, benzanthrone, sulfide vat dyes (blue BO, black BB and brown), etc. In addition, hydrogenated phenanthrene can be obtained by hydrogenating synthetic tanning agents and phenanthrene in the plastics industry under high temperature and pressure, which is a fuel for advanced jet aircraft.
2. Used as a stabilizer for high and low disinfection pesticides and explosives such as smokeless gunpowder.

 微信扫一扫打赏
微信扫一扫打赏

