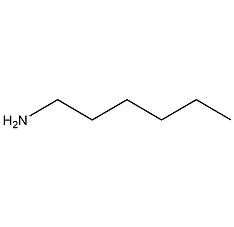
Structural formula
| Business number | 0333 |
|---|---|
| Molecular formula | C6H15N |
| Molecular weight | 101.19 |
| label |
1-Aminohexane, n-hexylamine, 1-Amino-n-hexane, 1-Hexylamine, 1-aminehexane, 1-Amine-n-hexane, 1-Hexylamine, n-Hexylamine, 1-Aminohexane, linear compound |
Numbering system
CAS number:111-26-2
MDL number:MFCD00008240
EINECS number:203-851-8
RTECS number:MQ4540000
BRN number:1731298
PubChem ID:None
Physical property data
1. Properties: colorless liquid with ammonia smell. [1]
2. Melting point (℃): -22.9[2]
3. Boiling point (℃): 131.4[3]
4. Relative density (water = 1): 0.77[4]
5. Relative vapor Density (air=1): 3.49[5]
6. Heat of combustion (kJ/mol): -4272.8[6]
7. Octanol/water partition coefficient: 1.52~2.34[7]
8. Flash point (℃): 8 (CC); 29 (OC) [8]
9. Ignition temperature (℃): 270[9]
10. Explosion limit (% ): 9.3[10]
11. Lower explosion limit (%): 2.1[11]
12. Solubility : Slightly soluble in water, miscible in ethanol and ether. [12]
Toxicological data
1. Acute toxicity: rat oral LD5O: 670mg/kg; rat inhalation LCLO: 500ppm/4H; rabbit transdermal LD5O: 420ul/kg;
2. Irritation:
p>
Rabbit skin open irritation test: 10mg/24H severe irritation.
Rabbit skin open irritation test: 500mg severe irritation.
Rabbit eye standard Drez eye dye test: 5mg moderately irritating.
Standard Drez eye dye test on rabbit eyes: 750ug/24H severe irritation.
3. Acute toxicity [13] LD50: 670mg/kg (rat oral); 420μl (323.4mg)/kg (rabbit oral Skin)
4. Irritation [14]
Rabbit transdermal: 500mg, severe irritation (open stimulation test).
Rabbit eye: 5mg, moderate irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects [15] This substance is harmful to the environment and should be treated with special care. Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 33.38
2. Molar volume (cm3/mol):131.2
3. Isotonic specific volume (90.2K): 299.8
4. Surface tension (dyne/cm): 27.2
5. Polarizability (10 -24cm3): 13.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 27.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances[17] Acids, acid anhydrides, strong oxidants
3. Polymerization hazards[18] No polymerization
4. Decomposition products[19] Ammonia
Storage method
Storage Precautions[20] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from methyl n-hexyl ketone oxime treated with phosphorus pentachloride. Treat methyl n-hexyl ketone oxime in diethyl ether with phosphorus pentachloride, then add water, mix well, and separate the layers. The N-acetyl n-ethylamine generated by the rearrangement is dissolved in diethyl ether, and the diethyl ether layer is separated. The ether is recovered by distillation, and the remaining oily liquid is hydrolyzed with a dilute potassium hydroxide solution and then distilled. Collect the 130-133°C fraction to obtain hexylamine.
Purpose
Used in organic synthesis. [21]

 微信扫一扫打赏
微信扫一扫打赏

