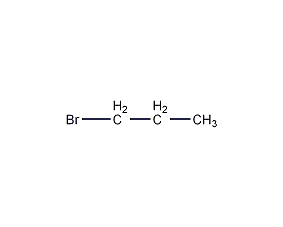
Structural formula
| Business number | 02U8 |
|---|---|
| Molecular formula | C3H7Br |
| Molecular weight | 122 |
| label |
1-bromopropane, Bromopropane, bromopropane, n-propyl bromide, n-Propane bromide, Bromide n-propane, propyl bromide, Bromopropane, n-Propyl bromide |
Numbering system
CAS number:106-94-5
MDL number:MFCD00000254
EINECS number:203-445-0
RTECS number:TX4110000
BRN number:505936
PubChem ID:None
Physical property data
1. Properties: colorless to yellow liquid with pungent odor. [1]
2. Melting point (℃): -110[2]
3. Boiling point (℃): 71[3]
4. Relative density (water = 1): 1.35[4]
5. Relative vapor Density (air=1): 4.3[5]
6. Saturated vapor pressure (kPa): 13.3 (18℃)[6]
7. Heat of combustion (kJ/mol): -2078.7[7]
8. Critical pressure (MPa): 5.39[8]
9. Octanol/water partition coefficient: 2.1[9]
10. Flash point (℃): -10[10]
11. Ignition temperature (℃): 490[11]
12. Explosion limit (%): 8.5[12]
13. Lower explosion limit (%): 4.6[13]
14. Solubility: insoluble Soluble in water, ethyl ether and carbon tetrachloride. [14]
15. Viscosity (mPa·s, 25ºC): 0.00495
16. Heat of evaporation (KJ/kg, 68.8ºC): 242.8
17. Heat of fusion (KJ/kg,-108.1ºC): 27.3
18. Heat of formation (KJ/mol, 298.16ºC): -124.47
19. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.12
20. Electrical conductivity (S/m, 25ºC): <2×10-8
21. Solubility (%, water, 30ºC): 0.230
22. Refractive index at room temperature (n20): 1.4343
23. Refractive index at room temperature (n25): 1.4317
24. Eccentricity factor: 0.285
25. Solubility parameter (J·cm -3)0.5: 18.083
26. van der Waals area (cm2·mol-1 ): 6.900×109
27. van der Waals volume (cm3·mol-1): 48.530
28. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -121.8
29. Liquid phase standard Hot melt (J·mol-1·K-1): 139.2
30. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -87.0
31. Gas phase standard entropy (J·mol-1·K-1): 330.81
32. Gas phase standard formation free energy (kJ·mol-1): -21.4
33. Gas phase standard hot melt (J·mol-1·K-1): 85.86
Toxicological data
1. Acute toxicity[15]
LD50: 3600mg/kg (rat oral); 4700mg/kg (orally in mice); 2900mg/kg (intraperitoneal cavity in rats)
2. Irritation No information available
Ecological data
1. Ecotoxicity[16]
LC50: 67.3mg/L (96h) (fathead minnow)
EC50: 208.9mg/L (24h) (Daphnia)
2. Biodegradability No data yet
3. Non Biodegradability[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, The degradation half-life is 17d (theoretical). At 25°C, when the pH value is 7, the hydrolysis half-life is 26 days (theoretical).
Molecular structure data
1. Molar refractive index: 23.67
2. Molar volume (cm3/mol): 91.2
3. Isotonic specific volume (90.2K ): 203.6
4. Surface tension (dyne/cm): 24.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 9.38
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 7.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Isomerization occurs when heated with aluminum tribromide to produce 2-bromopropane. Pyrolysis by heating or contact with flame produces toxic bromide gas. In case of fire, water, foam fire extinguishing agent, carbon dioxide, dry chemical fire extinguishing agent, carbon tetrachloride, etc. can be used to extinguish the fire.
2. Stability[18] Stable
3. Incompatible substances[19] Strong oxidants, strong bases, potassium, sodium, magnesium
4. Conditions to avoid contact [20] Heat
5. Polymerization hazard[21] No polymerization
6. Decomposition products[22] Hydrogen bromide
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. It should be stored separately from oxidants, alkalis, active metal powders, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
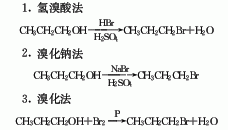
1. Obtained from the reaction of n-propanol and hydrobromic acid. Add hydrobromic acid to concentrated sulfuric acid, then add n-propanol, and heat to reflux for 0.5h. Evaporate all the generated bromopropane at 70-75°C, then wash with concentrated hydrochloric acid, and then neutralize with sodium carbonate to pH 7. Dry with anhydrous sodium sulfate, filter, and distill the filtrate. Collect the 69-74°C fraction to obtain propane bromide.
2. Obtained from the reaction of n-propanol and sodium bromide. Heat n-propanol, water and sodium bromide together to reflux, add sulfuric acid dropwise at 69-72°C, complete the addition, and continue refluxing for 2 hours. Distill, collect the distillate at 68-100°C, wash with sodium carbonate solution until neutral, distill again, collect the distillate at 68-76°C, which is propane bromide. In addition, propane bromide can also be prepared by reacting n-propanol with bromine in the presence of red phosphorus.
3. Preparation method:
p>
![]()
Add 48% hydrogen to the reaction bottle 500g bromic acid, cool in water bath, slowly add 150g (82mL) concentrated sulfuric acid while stirring, then add 144g (2.4mol) n-propanol (2), slowly heat, and add 65mL concentrated sulfuric acid dropwise from the dropping funnel, while evaporating The resulting 1-bromopropane is produced. The connecting tube extends into the receiving bottle under the water surface, and the receiving bottle is cooled with ice water. Stop the reaction when no bromine-free propane evaporates. The crude propane bromide in the distillate was separated, washed successively with water, 5% sodium carbonate solution, water, and dried over anhydrous calcium chloride. Heating and distilling in a water bath, collecting fractions at 70-72°C to obtain 255g of 1-bromopropane (1)①, with a yield of 86%. Note: ① 1-bromopropane can also be prepared by reacting sodium bromide, sulfuric acid and propanol. [25]
Purpose
1. Used in the manufacture of medicines, pesticides, dyes, spices, etc. It is also used as a raw material for Grignard reagent and an alkylating agent for aromatic compounds, and as an intermediate for the drugs propylthiamine and probenecid.
2. Used as solvents and laboratory reagents. [24]

 微信扫一扫打赏
微信扫一扫打赏

