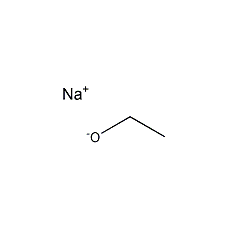
Structural formula
| Business number | 03U1 |
|---|---|
| Molecular formula | C2H5ONa |
| Molecular weight | 68.06 |
| label |
Sodium ethanol salt, sodium ethoxide, sodium ethoxide, Sodium ethylate, Caustic alcohol, catalyst |
Numbering system
CAS number:141-52-6
MDL number:MFCD00012417
EINECS number:205-487-5
RTECS number:None
BRN number:3593646
PubChem number:24886021
Physical property data
1. Physical property data:
1. Appearance: white or slightly yellow hygroscopic powder
2. Density (g/mL, 25/4℃): 0.868
3. Melting point (ºC): >300
4. Refractive index (nD20): 1.385
5. Flash point (℃): 30
6. Boiling point (ºC): 91
7. Solubility: It quickly decomposes into sodium hydroxide and ethanol when exposed to water, and is soluble in absolute ethanol without decomposition. Insoluble in benzene
Toxicological data
None yet
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: strong oxidants, acids, water
2.Non-toxic, it is a strong organic base and will not When exposed to humid air, sodium hydroxide is generated and becomes highly corrosive. When in contact with skin or eyes, it is highly corrosive and irritating. Direct contact with skin should be avoided. And should be equipped with necessary protective equipment, such as glasses, gloves and work clothes.
3.Decomposes when exposed to water. Strongly alkaline.
Storage method
1. Store in a cool, dry place and away from wind.
2.Seal and package in iron drums, 200kg or 160kg per drum. During storage and transportation, it must be fireproof, waterproof and moisture-proof. Store and transport according to regulations on flammable and toxic chemicals.
Synthesis method
1. Three-way azeotropic method of benzene, ethanol and water. Dissolve solid sodium hydroxide in ethanol and pure benzene solution.� or cyclohexane and ethanol solution), heated to reflux, and dehydrated through continuous reaction in a tower reactor until the total alkali content and free alkali reach the standard. The ternary azeotropic mixture of benzene, ethanol and water is evaporated from the top of the tower, and the ethanol solution of sodium ethoxide is obtained from the bottom of the tower.
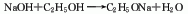
2. Metal sodium method consists of metal It is obtained by the reaction of sodium and absolute ethanol.
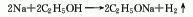
Purpose
1. Used as a strong alkaline catalyst, ethoxylating agent, and as a coagulant and reducing agent in organic synthesis. Pharmaceutical products using sodium ethoxide include phenybital, phenylbutazone, primidone, methyldopa, tetracaine hydrochloride, amoxicillin, triamterene, pyrimethamine, and pifril, etc. In addition, it is also used in pesticides and analytical reagents.
2. Mainly used in the pharmaceutical and pesticide industries.
3. Used as a strong alkaline catalyst, ethoxylating agent and reducing agent for organic synthesis, pharmaceutical synthesis, etc. Organic Synthesis. 4.Alkalizing agent. Used for nucleophilic substitution, condensation reactions under alkaline conditions (such as ester condensation, Michael condensation, etc.), dehydrohalogenation, etc.

 微信扫一扫打赏
微信扫一扫打赏

