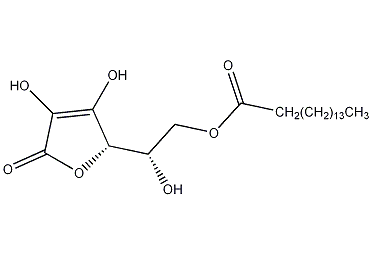
Structural formula
| Business number | 03RQ |
|---|---|
| Molecular formula | C22H38O7 |
| Molecular weight | 414.53 |
| label |
L-Ascorbyl Palmitate, 6-O-Palmitoyl-L-ascorbic acid, L-Ascorbyl palmitate, Ascorbic acid 6-palmitate, Antioxidants, food additives, vitamins, Nutrition supplements |
Numbering system
CAS number:137-66-6
MDL number:MFCD00005377
EINECS number:205-305-4
RTECS number:CI7671040
BRN number:96552
PubChem number:24857513
Physical property data
1. Properties: white or slightly yellow powder with a slight citrus smell. Sensitive to light.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 113
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): [a] 20/D+22.9°(c=2, in methanol).
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: 1g dissolves in about 4.5ml ethanol, extremely Slightly soluble in water and vegetable oil.
Toxicological data
Acute toxicity data: Mouse oral LD50: 25mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 109.62
2. Molar volume (cm3/mol): 360.2
3. Isotonic specific volume (90.2K): 959.9
4. Surface tension (3.0 dyne/cm): 50.4
5. Polarizability (0.5 10-24cm3): 43.45
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 6.1
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 18
5, Number of tautomers: 8
6, Topological molecular polar surface area (TPSA): 113
7. Number of heavy atoms: 29
8. Surface charge: 0
9. Complexity: 515
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
Sealed packaging. Store in a cool, dry warehouse away from high temperatures, light and oxidation. Do not store together with poisonous or polluting chemicals.
Synthesis method
1. Place 64g of palmitic acid in a heated dropping funnel to keep it in a molten state; add 200mI. Put thionyl chloride into a flask, heat it so that its vapor enters the reaction column equipped with porcelain ring packing through the conduit, and reacts with the palmitic acid dripped from the upper part of the column. The unreacted thionyl chloride condenses through the branch pipe at the upper part of the reaction column. Then return to the flask; after the palmitic acid is added dropwise, react for 30 minutes, remove a small amount of thionyl chloride by distillation under normal pressure, and collect 58.6g of colorless transparent liquid at 150~152°C (2~3kPa) under reduced pressure distillation, with a yield of 86% ( Calculated as palmitic acid).
2. Add 26.5mL to the flask. Dimethylformamide, add 2g of HCl gas at 0°C, then add 9.69g of crystallized ascorbic acid and 13.3mL. Dichloromethane, the reaction solution is clear at this time; slowly drop 15.2 mL of palmitoyl chloride at 0°C. After the dropwise addition is completed, react for 18 hours, then raise the temperature to 20°C and react for 30 minutes. Add the reactants to 100 mL. Ethyl acetate and 200 mL. Stir and wash in water for 2 hours, filter with suction, wash with water (50 mL×3) three times, and dry under vacuum at 50°C for 18 hours to obtain 17.5 g of white powder with a yield of 84.3%.
3.Chlorination of palmitic acid produces palmitoyl chloride, which is then esterified with ascorbic acid, filtered and dried to obtain ascorbyl palmityl ester.
Purpose
1. It has antioxidant and antioxidant synergistic properties and can be used as an antioxidant or synergist. 0.01% L-ascorbyl palmitate can extend the shelf life of most vegetable oils. Adding 0.01% to soybean oil has a better antioxidant effect than 0.02% of BHA and BHT. It has strong ability to protect fried food oil and fried food, preventing color and volatile odor caused by frying.
2.As an antioxidant. It is widely used for antioxidant preservation of various foods and beverages such as oils, dairy products, cosmetics, health products, baby food, aquatic products, puffed foods, fried foods, meat products, nuts, fruits, etc. For oil-containing foods, instant noodles, and hydrogenated vegetable oil, the maximum usage amount is 0.2g/kg; for infant formula foods, the maximum usage amount is 0.01g/kg (calculated as ascorbic acid in oil and fat).

 微信扫一扫打赏
微信扫一扫打赏

