Background and overview[1]
N-nonylbenzene is an organic intermediate that can be used in laboratory research and development and chemical and pharmaceutical synthesis processes. It can be prepared from 5-allyltetradec-1-en-5-ol in two steps.
Preparation[1]
Step 1
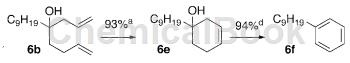
5-allyltetradec-1-en-5-ol (6b): Allylmagnesium bromide (1M Et2O solution, 0.83mL, 0.83mmol ) was added dropwise to a stirred and cooled (0°C) solution of Tetradec-1-ene-5-one (0.1454g, 0.69mmol) in THF (4mL). Leave the cold bath in place without recharging and continue stirring for 2 hours. The mixture was quenched with saturated aqueous NH4Cl solution and extracted with CH2Cl2. The combined organic extracts were washed with brine, dried (Na2SO4) and evaporated. Flash chromatography of the residue on silica gel (1 × 30 cm) using 10% EtOAc-hexane afforded 6b (0.1581 g, 91%) as an oil.
Step 2
1-Nonylcyclohex-3-enol (6e). A solution of 6b (0.14 g, 0.56 mmol) in CH2Cl2 (11 mL) was degassed with a stream of Ar gas for 30 min. Grubbs I catalyst (0.023g, 0.028mmol) was added and Ar flow was continued for 15 minutes. The mixture was then refluxed under Ar static pressure overnight, cooled, and filtered through flash chromatography silica gel (1 × 5 cm) using CH2Cl2. The solvent was evaporated and the residue was flash chromatographed on silica gel (1.5 × 20 cm) using 10% EtOAc-hexane to afford 6e (0.1149 g, 93%) as an oil.
Step 3
1-Nonylbenzene (6f), add TsOH.H2O (0.0064g, 0.033mmol) and DDQ (0.0076g, 0.033mmol) to 6e (0.0075g, 0.033mmol) ) in a stirred solution in PhH (1.5 mL). The mixture was refluxed for 30 min, then cooled to room temperature and filtered through flash chromatography silica gel (0.5 x 5 cm) using petroleum ether. The filtrate was evaporated to give n-nonylbenzene (0.0064 g, 94%).
Main reference materials
[1] Clive D L J , Pham M P . Conversion of Weinreb Amides into Benzene Rings Incorporating the Amide Carbonyl Carbon[J]. The Journal of Organic Chemistry, 2009, 74(4):1685-1690.

 微信扫一扫打赏
微信扫一扫打赏

