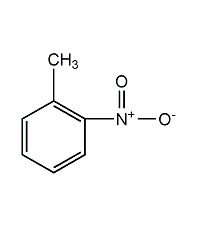
Structural formula
| Business number | 01ZY |
|---|---|
| Molecular formula | C7H7NO2 |
| Molecular weight | 137 |
| label |
2-Nitrotoluene, 1-Methyl-2-nitrobenzene, 2-Nitrotoluene, 1-Methyl-2-nitrobenzene, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:88-72-2
MDL number:MFCD00007157
EINECS number:201-853-3
RTECS number:XT3150000
BRN number:1907580
PubChem number:24869607
Physical property data
1. Properties: slightly yellow liquid[1]
2. Melting point (℃): -9.5[2]
3. Boiling point (℃): 222[3]
4. Relative density (water=1): 1.16[4]
5. Relative vapor density (air=1): 4.72[5]
6. Saturated vapor pressure (kPa): 0.03 (25℃)[6]
7. Heat of combustion (kJ/mol): -3593.1[7]
8. Critical pressure ( MPa): 3.8[8]
9. Octanol/water partition coefficient: 2.3[9]
10. Flash point (℃): 106 (CC); 95 (OC) [10]
11. Ignition temperature (℃): 305[11]
12. Explosion upper limit: No information yet
13. Explosion lower limit (%): 2.2[12]
14. Solubility: Insoluble in water, soluble in ethanol, ether, benzene, acetone, chloroform and petroleum ether. [13]
Toxicological data
1. Acute toxicity[14]
LD50: 891mg/kg (rat oral)
LC50 : 790mg/m3 (rat inhalation)
2. Irritation No information available
Ecological data
1. Ecotoxicity[15]
LC50: 65mg/L (96h) (zebrafish); 29mg/L (48h) (Tall fish); 88mg/L (48h) (medaka); 37.1mg/L (96h) (fathead minnow); 8.8mg/L (48h) (water flea)
IC50: 3.1~28mg/L (72h) (algae)
2. Biodegradability[16] MITI-I test, initial concentration 100ppm , the sludge concentration is 30ppm, and the degradation is 0.5% after 2 weeks.
3. Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 37.62
2. Molar volume (cm3/mol): 117.5
3. Isotonic specific volume (90.2K ): 300.4
4. Surface tension (dyne/cm): 42.6
5. Polarizability (10-24cm3): 14.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 45.8
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 130
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[17] Stable
2. Incompatible substances[18] Strong oxidizing agent, strong reducing agent, strong alkali
3. Conditions to avoid contact[19] Heating
4. Polymerization hazard[20] No polymerization
5. Decomposition products[21] Nitrogen oxides
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Toluene is nitrated with mixed acid to produce mixed nitrotoluene, which is mainly o-nitrotoluene (accounting for about two-thirds) and p-nitrotoluene (accounting for about one-third). The pure product can be obtained after separation . Add toluene into the reactor, cool it to below 25°C, add the prepared mixed acid (i.e. 25-30% nitric acid, 55-58% sulfuric acid and 20-21% water), and adjust the temperature not to exceed 50°C. Stir continuously for 1-2 hours, then let it stand for 6 hours. Separate the generated nitrotoluene, wash with water and alkali to remove unreacted toluene and aliphatic compounds. The composition of the crude nitrotoluene product is o-nitrotoluene 55-60 %, meta position 2-5%, counter position 35-40%. The yield is 90-95%. The isomers can be separated by crude distillation and crystallization using the difference between boiling point and melting point. That is, the crude nitrotoluene is first subjected to vacuum distillation to separate most of the o-nitrotoluene, and the remaining fraction containing more p-nitrotoluene is separated by vacuum distillation, cooled, crystallized, and separated to obtain Finished product. High-boiling tar-like substances remain in the still. Meta-nitrotoluene is contained in the mother liquor after separation of the para-mer, and is obtained by distillation after repeated accumulation. The purity of ortho- and para-nitrotoluene can reach 98% and 99% respectively. The domestic process involves two pots connected in series, with the reaction temperature of the main pot being 40-45°C and the secondary pot being 50-55°C. The preparation of mixed acid is roughly similar, 26-28% nitric acid, 56-57% sulfuric acid, and 16-18% water. Raw material consumption quota: toluene (98%) 800kg/t, nitric acid (98%) 470kg/t, sulfuric acid (92.5%) 450kg/t, caustic soda (42%) 100kg/t.
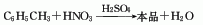
Purpose
1. Mainly used in the production of o-toluidine and toluidine, which are important raw materials for dyes, coatings, plastics and medicines. In the pharmaceutical industry, it is used to produce nifedipine, Tongjingning, imipramine hydrochloride, bromexamine hydrochloride, diclofenac penicillin sodium, etc. Also used as solvent.
2. Used in the synthesis of various dyes. [23]

 微信扫一扫打赏
微信扫一扫打赏

