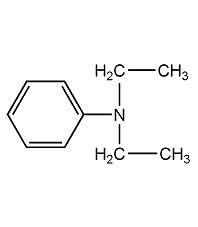
Structural formula
| Business number | 023X |
|---|---|
| Molecular formula | C10H15N |
| Molecular weight | 149.23 |
| label |
Diethylaminobenzene, N,N-diethylaniline, Diethylamino benzene, N,N-diethyl aniline, deacidifying agent, metal preservatives, polyester resin curing agent, Polymerization accelerator for vinyl compounds, Stabilizer for acrylonitrile |
Numbering system
CAS number:91-66-7
MDL number:MFCD00009042
EINECS number:202-088-8
RTECS number:BX3400000
BRN number:742483
PubChem number:24859178
Physical property data
1. Properties: colorless to yellow oily liquid with a special odor. [1]
2. Melting point (℃): -38.8[2]
3. Boiling point (℃): 215~216[3]
4. Relative density (water=1): 0.93 (25℃)[4]
5. Relative vapor density (air=1): 5.2[5]
6. Saturated vapor pressure (kPa): 0.31 (65℃)[6 ]
7. Octanol/water partition coefficient: 3.31[7]
8. Flash point (℃): 85 (CC )[8]
9. Ignition temperature (℃): 630[9]
10. Solubility: Soluble in water, slightly soluble in ethanol, ether, chloroform, soluble in acids. [10]
11. Melting point (ºC, stable type): -21.3
12. Melting point (ºC, unstable type): -34.4
13. Refractive index (20ºC): 1.5421
14. Viscosity (mPa·s, 11ºC): 3.251
15. Viscosity (mPa·s, 25ºC ): 1.9298
16. Flash point (ºC, closed): 85
17. Flash point (ºC, open): 80
18. Fire point ( ºC): 330
19. Heat of evaporation (KJ/kg, 215.2ºC): 310.4
20. Heat of fusion (KJ/mol): 8489
21. Heat of formation (KJ/mol, liquid): -16.29
22. Heat of combustion (KJ/mol, 20ºC): 6077.6
23. Heat of combustion (KJ/mol, 25ºC): 6066.7
24. Specific heat capacity (KJ/(kg·K), 28.83ºC, constant pressure): 1.84
25. Thermal conductivity (W/(m·K) ),20ºC): 0.136
Toxicological data
1. Acute toxicity[11]
LD50: 782mg/kg (rat oral)
LC50: 1920mg/m3 (rat inhalation, 4h)
2. Irritation No information available
Ecological data
1. Ecotoxicity[12] LC50: 16.8mg/L (48h) (medaka)
2. Biodegradability No data yet
3. Non-biodegradability[13] In the air, when the hydroxyl radical concentration is 5.00×105 units/cm3, the degradation half-life is 2h (theoretical).
4. Bioconcentration[14] BCF: 44~161 (carp, contact concentration 0.2mg/L, contact time 8 weeks); 17~125 (carp, exposure concentration 0.02mg/L, exposure time 8 weeks)
Molecular structure data
1. Molar refractive index: 49.23
2. Molar volume (cm3/mol): 160.4
3. Isotonic specific volume (90.2K ): 388.8
4. Surface tension (dyne/cm): 34.5
5. Polarizability (10-24cm3): 19.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 3.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 91
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: N,N-diethylaniline forms salts with acids and quaternary ammonium salts with alkyl halides. N-formanilide is generated when oxidized with manganese dioxide. When oxidized with chromic acid, it decomposes to produce acetic acid and ammonia. When oxidized with hydrogen peroxide, N,N-diethylaniline suboxides are generated.
2. Stability[15] Stable
3. Incompatible substances[16] Strong oxidants, strong acids
4. Conditions to avoid contact[17] Light
5. Hazards of aggregation[18] No aggregation
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Ethyl chloride method: Obtained from the reaction of aniline and ethyl chloride. 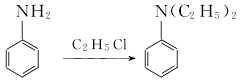 Due to the exothermic reaction process, heating was stopped when the system reaction temperature was 120°C and 1.18MPa; the reaction was maintained. In 3 hours, the temperature in the pot can reach 215-230℃ and the pressure is 4.4-4.5MPa. After the pressure is released, the water vapor is distilled to obtain the crude product. According to the content of monoethylaniline in the crude product, phthalic anhydride is added for acylation, and then steam distillation is performed for the second time. The distillate is the finished product after removing water. Raw material consumption (kg/t): aniline 645, ethyl chloride (95%) 1,473, caustic soda (42%) 1,230, phthalic anhydride 29.
Due to the exothermic reaction process, heating was stopped when the system reaction temperature was 120°C and 1.18MPa; the reaction was maintained. In 3 hours, the temperature in the pot can reach 215-230℃ and the pressure is 4.4-4.5MPa. After the pressure is released, the water vapor is distilled to obtain the crude product. According to the content of monoethylaniline in the crude product, phthalic anhydride is added for acylation, and then steam distillation is performed for the second time. The distillate is the finished product after removing water. Raw material consumption (kg/t): aniline 645, ethyl chloride (95%) 1,473, caustic soda (42%) 1,230, phthalic anhydride 29.  2. Bromoethane method: In a 500ml container equipped with an electric stirrer, thermometer and reflux condenser In the four-neck reaction flask, add 11ml (0.12mol) aniline, 15.75ml (0.21mol) bromoethane, 1.00g benzyltriethylammonium chloride, 35ml sodium hydroxide solution with a mass fraction of 0.35, and heat the reaction flask The internal temperature is 45°C, and the reaction is stirred for 3 hours under normal pressure; cool to room temperature, pour the reaction solution into a separatory funnel, and let stand for layering. Separate the oil and water layers; extract the water layer three times with 30 ml of diethyl ether, and mix the extract with the oil layer. Dry with anhydrous magnesium sulfate for 3 hours, filter and evaporate the ether. The residue was treated with an equal volume of acetic anhydride overnight to remove the free secondary amine. Then add excess hydrochloric acid solution with a mass fraction of 0.1, wash until it is acidic (PH=1-2), separate acetyl-N-ethylaniline, and alkalinize to PH=11-12 with a sodium hydroxide solution with a mass fraction of 0.25. , let stand to separate the two layers of oil and water. The aqueous layer was extracted twice with 30 ml of diethyl ether. Mix the extract with the oil layer, dry with anhydrous magnesium sulfate for 3 hours, evaporate the ether and then distill under reduced pressure. Collect the fraction with a boiling point of 62-66°C/400pa to obtain 11.18g of N, N-diethylaniline, with a yield of 70.4%. . 3. Refining method: The main impurity is N-ethylaniline, which can be separated by distillation or acylation of N-ethylaniline. Since the acylate of N-ethylaniline has low volatility, N,N-di Ethylaniline is distilled.
2. Bromoethane method: In a 500ml container equipped with an electric stirrer, thermometer and reflux condenser In the four-neck reaction flask, add 11ml (0.12mol) aniline, 15.75ml (0.21mol) bromoethane, 1.00g benzyltriethylammonium chloride, 35ml sodium hydroxide solution with a mass fraction of 0.35, and heat the reaction flask The internal temperature is 45°C, and the reaction is stirred for 3 hours under normal pressure; cool to room temperature, pour the reaction solution into a separatory funnel, and let stand for layering. Separate the oil and water layers; extract the water layer three times with 30 ml of diethyl ether, and mix the extract with the oil layer. Dry with anhydrous magnesium sulfate for 3 hours, filter and evaporate the ether. The residue was treated with an equal volume of acetic anhydride overnight to remove the free secondary amine. Then add excess hydrochloric acid solution with a mass fraction of 0.1, wash until it is acidic (PH=1-2), separate acetyl-N-ethylaniline, and alkalinize to PH=11-12 with a sodium hydroxide solution with a mass fraction of 0.25. , let stand to separate the two layers of oil and water. The aqueous layer was extracted twice with 30 ml of diethyl ether. Mix the extract with the oil layer, dry with anhydrous magnesium sulfate for 3 hours, evaporate the ether and then distill under reduced pressure. Collect the fraction with a boiling point of 62-66°C/400pa to obtain 11.18g of N, N-diethylaniline, with a yield of 70.4%. . 3. Refining method: The main impurity is N-ethylaniline, which can be separated by distillation or acylation of N-ethylaniline. Since the acylate of N-ethylaniline has low volatility, N,N-di Ethylaniline is distilled.  4. Diethyl phosphite method: from aniline and diethyl phosphite under normal pressure Obtained by reaction.
4. Diethyl phosphite method: from aniline and diethyl phosphite under normal pressure Obtained by reaction.
Purpose
1. Used as a curing and accelerator for unsaturated polyester adhesives. It can also be used as an accelerator for vinyl compound polymer rubber and anaerobic adhesives, as well as plastic curing agents, chemical reaction deacidification agents, and metal preservatives. wait. It is also used as a raw material for manufacturing triphenylmethane dyes, azo dyes, basic dyes, tetraethyl ketone, pharmaceuticals and color developers.
2. Used in the synthesis of dyes and their intermediates, and also in the manufacture of medicines. [20]

 微信扫一扫打赏
微信扫一扫打赏

