Background and overview[1]
P-substituted acetophenones in aromatic ketones, including p-tert-butyl acetophenone, are an important class of intermediates that can be used in the production of fine and specialty chemicals such as drugs and fragrances. In industry, it is often produced by Friedel-Crafts acylation reaction using aromatic compounds and carboxylic acid derivatives as raw materials under the action of catalysts. Commonly used catalysts are Lewis acids (such as AlCl3) or strong inorganic acids (such as HF and H2SO4). Due to complexing with acyl groups, the catalyst often needs to be doubled in excess, and the excess catalyst often leads to the production of a large amount of non-metallic and acid residues in industrial post-processing. At the same time, environmental pollution and equipment corrosion are serious during the production process.
For example, the existing production method of p-methoxyacetophenone mainly uses an equivalent amount of aluminum trichloride to catalyze the reaction with acetyl chloride to generate p-methoxyacetophenone. The catalyst aluminum trichloride cannot Recycling produces a large amount of ice hydrolysis wastewater containing aluminum trichloride with high COD and salt content. And a large amount of hydrogen chloride waste gas is produced during the reaction. Therefore, there is a need to find environmentally friendly, cheap, easily recyclable, and reusable green catalysts.
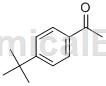
Preparation[2]
The preparation of p-tert-butyl acetophenone is as follows: add 2-(4-(tert-butyl)phenyl)propan-2-ol (59.4mg, 0.3mmol), AgNO3 (1.6mg, 3mol%), Bi(OTf)3 (6mg, 3mol%) and K2S2O8 (245.8mg, 3eq). Finally, 2wt% DAPGS-750-M aqueous solution (0.6ml, 0.5M) was added, and then the reaction bottle was capped and stirred. After the reaction solution is stirred, extract it three times with ethyl acetate. Combine the organic phases extracted several times into a 50mL eggplant-shaped flask. Use a Heidolph rotary evaporator with a rotation speed of 120 rpm, a temperature of 38°C, and a vacuum of 0.1Mpa. About 5 minutes, and then use 200 mesh column chromatography silica gel to perform column chromatography. The developing solvent is petroleum ether: ethyl acetate = about 20:1, and the target compound p-tert-butyl acetophenone is separated. (42.8mg, yield 81%, purity after HPLC analysis is 98%, the appearance, signal, noise, etc. of the NMR spectrum can also reflect the extremely high purity of the product).
1HNMR (600MHz, CDCl3) δ7.92–7.87 (m, 2H), 7.49–7.45 (m, 2H), 2.58 (s, 3H), 1.34 (s, 9H).

Main reference materials
[1] CN201810398828.5 A production process for solid acid catalyzed synthesis of para-substituted acetophenones compounds
[2] CN201810144871.9 A new method for preparing ketones by aqueous phase oxidation of tertiary alcohols

 微信扫一扫打赏
微信扫一扫打赏

