Overview[1][2]
4-Butylbenzene-1-sulfonyl chloride is an organic intermediate that can be prepared from butylbenzene in one step.
Preparation[1]
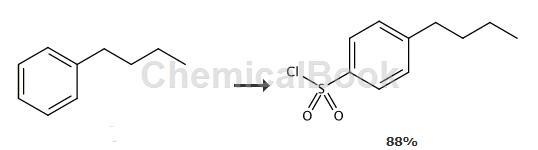
To a solution of butylbenzene (4.13g, 30.8mmol) in CHCl3 (50mL) was added chlorosulfonic acid (17mL, 29.8g, 256mmol) and the mixture was stirred at room temperature for 20 hours . The mixture was poured onto ice (200 mL) and extracted with EtOAc (3 x 100 mL). The combined extracts were washed with water, NaHCO3 solution and water, dried (Na2SO4), and concentrated in vacuo. The yellow oily residue (yield 88%) was used in the next step without further purification. 1H NMR (300MHz, CDCl3) δ0.94 (t, 3H, J = 7Hz), 1.34-1.41 (m, 2H), 1.62-1.67 (m , 2H), 2.73 (t, 2H, J = 8Hz), 7.41 (d, 2H, J = 8Hz), 7.94 (d, 2H, J = 8Hz).
Application [2-3]
Can be used in acylation reactions, such as the preparation of sulfonamides.

A solution of the compound amine (150 mg, 1.11 mmol) in pyridine (4 mL) was treated with 4-phenylbenzenesulfonyl chloride (279.2 mg, 1.11 mmol). The mixture was stirred at room temperature overnight (approximately 18 hours). Water was added to the solution and extracted with dichloromethane (3 times). The combined organic phases were washed with 3M HCl and concentrated to afford 205 mg of the desired product as a solid (1H NMR confirmed, 58.6% yield).
It can also be used to prepare sulfonyl alkynyl groups.
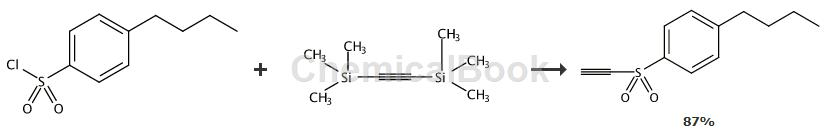
General procedure: Combine sulfonyl chloride (1.1 equiv) and anhydrous AlCl3 (1.1 equiv) in CH2Cl2 (1.3 -1.7 mL/mmol) was stirred under Ar at room temperature for 20 min. The resulting solution was added via cannula to ice-cooled BTMSA (68, 1.0 equiv) in CH2Cl2 (0.7-0.9 mL/mmol) over 30 min. in solution. removeIce bath, the mixture was stirred at room temperature under Ar for 17-19 hours. Stop the reaction by carefully pouring the mixture into 1 M HCl (2.5 mL/mmol) and ice (80 mg/mmol). The organic phase (organic phase) was separated, dried over Na2SO4 and concentrated in a rotary evaporator.
Main reference materials
[1] From PCT Int. Appl., 2009129267, 22 Oct 2009
[2] From U.S. Pat. Appl. Publ., 20140113895, 24 Apr 2014
[3] Structure-Based Design and Synthesis of Inhibitors for Plasmepsine and Falcipaine, By Zuercher, Martina From No pp.; 2009

 微信扫一扫打赏
微信扫一扫打赏

