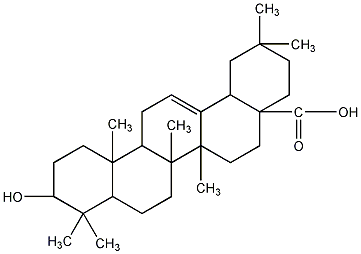
Structural formula
| Business number | 05A6 |
|---|---|
| Molecular formula | C30H48O3 |
| Molecular weight | 456.70 |
| label |
Oleanolic acid, Angelica sinensis is sour, Oleanolic acid, broad spectrum antibacterial drugs |
Numbering system
CAS number:508-02-1
MDL number:MFCD00064914
EINECS number:208-081-6
RTECS number:None
BRN number:None
PubChem number:24898021
Physical property data
1. Properties: White crystalline powder
2. Melting point (ºC): 310
3. Boiling point (ºC, normal pressure): 554
4. Specific optical rotation (º): [α] 20D is 83.3°
5. Heat of combustion (KJ/mol): 95.9
6. Solubility: Almost insoluble In water, slightly soluble in ethanol and chloroform.
Toxicological data
Acute toxicity: rat intravenous LD50: >2gm/kg, no detailed instructions except lethal dose;
Rat intraperitoneal LD50: >2gm/kg, no detailed instructions except lethal dose ;
Mouse oral LC50: >2gm/kg, no details except lethal dose;
Mouse intraperitoneal LC50: 1500mg/kg, no details except lethal dose illustrate.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 133.57
2. Molar volume (cm3/mol): 414.9
3. Isotonic specific volume (90.2K ): 1077.0
4. Surface tension (dyne/cm): 45.4
5. Polarizability (10-24cm3): 52.95
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 7.5
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 57.5
7. Number of heavy atoms: 33
8. Surface charge: 0
9. Complexity: 885
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 8
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications. It will not decompose and avoid contact with oxides.
Storage method
Brown glass bottle with light-sealed packaging. Store at low temperature and dry place.
Synthesis method
1 Alcohol extraction method
Cut 100g of wood root bark into small pieces or filaments, add 500ml of 70% ethanol and reflux for extraction three times, about 3 hours each time, combine the alcohol extracts; then distill and recover Most of the ethanol, then add sulfuric acid to make the concentration 15%, reflux for 2 hours, let it cool and then filter. Wash the filter cake with water 3 times, boil it with 3% NaOH solution 2 times, boil it once with water, and filter after each boiling time. The crude gray sodium salt product can be obtained. Add about 8 times the amount of more than 85% ethanol and 0.5% activated carbon to the crude product, reflux for 40 minutes, filter, adjust the filtrate to pH=3~4 with HCl, leave it overnight, filter, wash the filter cake with distilled water until there is no chloride ion, and dry it. Premium oleanolic acid.
2 Water extraction method
Crush the root bark and decoct it with water three times for about 1 hour each time. Combine the water decoctions and concentrate them into a thin paste. Then add concentrated sulfuric acid and stir continuously to make the concentration reach 20%. Heat for about 1 hour. After cooling, add water to dilute and filter. After washing the filter cake with water, boil it twice with 3% NaOH solution and once with water. Filter after each boiling time. , to obtain crude sodium salt. Add about 8 times the amount of more than 85% ethanol and 0.5% activated carbon to the crude product, reflux for 40 minutes, filter, adjust the filtrate to pH=3~4 with HCl, leave it overnight, filter, wash the filter cake with distilled water until there is no chloride ion, and dry it. Get this product.
Purpose
1. Oleanolic acid is a broad-spectrum antibacterial drug that is clinically used to treat bronchitis, pneumonia, acute tonsillitis, periodontitis, bacillary dysentery, acute gastroenteritis, and urinary system infections. In addition, it is also used clinically to treat acute hepatitis.

 微信扫一扫打赏
微信扫一扫打赏

