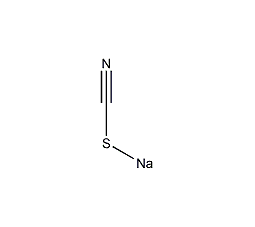
Structural formula
| Business number | 05KE |
|---|---|
| Molecular formula | NaSCN |
| Molecular weight | 81.07 |
| label |
sodium thiocyanide, Sodium isothiocyananate, Sodium rhodanate, Sodium rhodanide, Sodium sulfocyanate, spinning solvent, film developing agent, defoliants, Herbicide |
Numbering system
CAS number:540-72-7
MDL number:MFCD00011123
EINECS number:208-754-4
RTECS number:XL2275000
BRN number:3594965
PubChem ID:None
Physical property data
1. Properties: white orthorhombic crystal or powder. Hygroscopic.
2. Density (g/cm3, 25/4℃): 1.735
3. Relative steam density (g/cm3 , air=1): Undetermined
4. Melting point (ºC): 287
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC) : Undetermined
12. Saturated vapor pressure (kPa, 55.1ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined Determined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol and acetone, soluble in water.
Toxicological data
Low toxicity, median lethal dose (rat, oral) LD50: 764mg/kg. Irritating. Thyroid damage occurs with chronic poisoning. After people take 30g orally, acute psychosis similar to schizophrenia will occur within 4 to 8 hours.
Maximum allowable concentration in air: 50mg/m3
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
Temporarily��
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 24.8
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 34.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. It decomposes when heated and releases toxic gases such as nitrogen and sulfur oxides.
2. The aqueous solution is neutral. It reacts with iron salts to form blood-red ferric thiocyanide. It does not react with ferrous salts. It reacts with concentrated sulfuric acid to form yellow sodium bisulfate. It reacts with cobalt salts to form dark blue thiocyanate. Cobalt reacts with silver salt or copper salt to form white silver thiocyanate precipitate or black copper thiocyanide precipitate. Easily deliquescent in air.
3. Staff should take good precautions. If they accidentally touch their skin or eyes, they should rinse them with plenty of water immediately. Solubility 142.6g/100g water (25℃), 225.6 (101.4℃).
Storage method
1. Store in a cool, dry and well-ventilated warehouse.
2. Keep away from fire and heat sources. Protect from direct sunlight.
3. Packaging and sealing. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Synthesis methodReact sodium cyanide with sulfur to generate sodium thiocyanate. After impurity removal, activated carbon decolorization, and filtration, the filtrate is evaporated, filtered, crystallized, and separated to obtain sodium thiocyanate. Its
NaCN+S→NaSCN
2. The arsenic-alkali by-product method uses arsenic alkali liquid to remove hydrogen sulfide from coke oven gas, generating sodium thiocyanide and sodium cyanide, which are converted into sulfur through the regeneration tower Sodium sulfate and sodium cyanate.
The above-mentioned arsenic alkali desulfurization waste liquid is treated with sulfuric acid to control the Ph value to 4.5 to precipitate arsenic sulfide and neutralize it with soda ash. At this time, the sodium thiocyanate content in the raw material liquid is 130-160 g/L, and the sulfur content is 130-160 g/L. Sodium sulfate 250~300 g/L, evaporated in a vacuum, crystallized at 15~16℃, separated sodium sulfate and sodium thiosulfate, dissolved the crude product in water, added sulfuric acid and kept at 90~100℃ for 2 hours to make the thiosulfate Sodium sulfate is converted into sodium sulfate, which is removed by filtration. The filtrate is added with alkali to remove iron, and barium hydroxide is added to remove residual sodium sulfate. After the upper layer is purified, the filtrate is then vacuum evaporated and centrifuged to obtain a product with a purity of more than 98% and containing sodium oxide. Sodium thiocyanate finished product with an iron (FeO) content of less than 0.0005%. The reaction is as follows:
3Na2S2O3+H2SO4→3Na2SO4+4S+H2O
Fe3++3OH-→Fe(OH)3↓
Na2SO4+Ba(OH)2→BaSO4↓+2NaOH3. Add an equal amount of NaOH to the NH4SCN aqueous solution, and evaporate in a porcelain evaporating dish until no NH3 is produced. Continue evaporation until crystals begin to precipitate. The resulting crystals are recrystallized with ethanol and acetone, and then recrystallized once with ethanol. Remove the mother liquor by centrifugation. Dry at 130°C. 4.Add 48% sodium hydroxide solution to the same mass of In ammonium thiocyanate solution, heat the reaction until ammonia gas is no longer produced and crystals precipitate: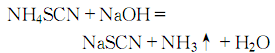 Cool, filter, and crystallize using ethanol and acetone respectively Recrystallize once each, spin dry, and dry at 130°C.
Cool, filter, and crystallize using ethanol and acetone respectively Recrystallize once each, spin dry, and dry at 130°C.
5.Extraction of sodium thiocyanide from tannin extract desulfurization liquid. After the tannin extract desulfurization liquid is clarified in the clarification tank, it enters the Na2S2O3 evaporation After the desulfurization liquid is evaporated to a certain density, it is put into the clarification tank for clarification, and filtered by the filter, and the filter residue is processed separately. The clear liquid enters the crystallization tank, where it is cooled with low-temperature water and slowly stirred. After the crystallization reaches the end, it is separated by a centrifuge. The filter cake is the Na2S2O3 product, and the separated liquid is used to extract the product NaCVS. The Na2S2O3 separated liquid is further clarified in the clarification tank. The clear liquid enters the crude NaCVS evaporator and is heated and evaporated. When it is concentrated to the point where crystals appear, it can be placed in a centrifuge for hot filtration. The separated liquid is returned to the desulfurization system or Apply. Crude NaCVS is put into the dissolving tank, dissolved with distilled water and heated. After the dissolved liquid passes through the clarification tank and filter, the clear liquid enters the refined NaCVS evaporator and is evaporated and concentrated until the crystallization appears, which is carried out by a centrifuge. After separation, the separated liquid is returned to the desulfurization system, and the solid is the NaCVS product.
Purpose
1. Used as polyacrylonitrile fiber spinning solvent, chemical analysis reagent, color film film developing agent, certain plant defoliants, and airport road herbicide. It is also used in pharmaceuticals, printing and dyeing, rubber processing, black nickel plating and the manufacture of artificial mustard oil.
2. Antifungal preservative, used in combination with hydrogen peroxide to preserve milk.�It has the effect of inhibiting mold. Our country stipulates that the maximum dosage per liter of raw milk is 2.0mL of 0.3% hydrogen peroxide, plus 16.76mg of sodium thiocyanate. Can be used in Heilongjiang and Inner Mongolia. If the area of use is to be expanded, the provincial health department must submit it to the Ministry of Health for review and approval and implement it in accordance with the relevant implementation specifications of the Ministry of Agriculture.
3. Used as analytical reagents, such as masking agents, precipitants, and color developers. Also used in printing, dyeing and pharmaceutical industries.
4.Used as a stabilizer for the viscosity of polyvinyl alcohol aqueous solution. It is also used as a solvent for polyacrylonitrile, color film developing agent, chemical analysis reagent, plant defoliant and rubber treatment agent.
5.Used for cyanide copper plating, cyanide silver plating and removal of nickel plating on copper and copper-tin alloy substrates It is mainly used as an additive in solutions such as black nickel plating and silver plating electrolyte.

 微信扫一扫打赏
微信扫一扫打赏

