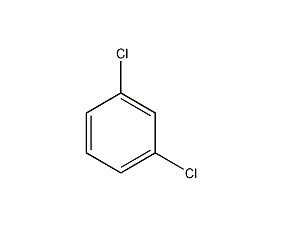
Structural formula
| Business number | 05L4 |
|---|---|
| Molecular formula | C6H4Cl2 |
| Molecular weight | 147 |
| label |
m-Dichlorobenzene, m-Dichlorobenzene, m-Phenylene dichloride, Metadichlorobenzene, Aromatic halogen derivatives |
Numbering system
CAS number:541-73-1
MDL number:MFCD00000573
EINECS number:208-792-1
RTECS number:CZ4499000
BRN number:956618
PubChem ID:None
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -24.8[2]
3. Boiling point (℃): 173[3]
4. Relative density (water = 1): 1.29[4]
5. Relative vapor Density (air=1): 5.08[5]
6. Saturated vapor pressure (kPa): 0.13 (12.1℃)[6]
7. Heat of combustion (kJ/mol): -2952.9[7]
8. Critical temperature (℃): 415.3[8]
9. Critical pressure (MPa): 4.86[9]
10. Octanol/water partition coefficient: 3.53 [10]
11. Flash point (℃): 72[11]
12. Ignition temperature (℃): 647 [12]
13. Explosion upper limit (%): 7.8[13]
14. Explosion lower limit (%) : 1.8[14]
15. Solubility: Insoluble in water, soluble in ethanol and ether, easily soluble in acetone. [15]
16. Viscosity (mPa·s, 23.3ºC): 1.0450
17. Flash point (ºC): 648
18. Heat of evaporation (KJ/mol, b.p.): 38.64
19. Heat of formation (KJ/mol, 25ºC, liquid): 20.47
20. Heat of combustion (KJ /mol, 25ºC, liquid): 2957.72
21. Specific heat capacity (KJ/(kg·K), 0ºC, liquid): 1.13
22. Solubility (%, water, 20ºC ): 0.0111
23. Relative density (25℃, 4℃): 1.2828
24. Refractive index at room temperature (n25): 1.5434
25. Solubility parameter (J·cm-3)0.5: 19.574
26. van der Waals area (cm2·mol-1): 8.220×109
27. van der Waals volume (cm3 ·mol-1): 87.300
28. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -20.7
29. Liquid phase standard hot melt (J·mol-1·K-1): 170.9
30. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): 25.7
31. Gas phase standard entropy (J·mol-1 ·K-1): 343.64
32. Gas phase standard free energy of formation (kJ·mol-1): 78.0
33. Vapor phase standard hot melt (J·mol-1·K-1): 113.90
Toxicological data
1. Acute toxicity: mouse intraperitoneal LD50: 1062 mg/kg, no detailed description except lethal dose;
2. Multiple dose toxicity data: rat oral TDLo: 1470 mg/kg /10D-I, Liver – changes in liver weight, total nutrient metabolism, calcium – enzyme inhibition, induced changes or alterations in blood or tissue levels – phosphatase;
RatOral TDLo: 3330mg/kg/90D-I, endocrine changes, changes in blood-serum components (such as tea polyphenols, bilirubin, cholesterol) Biochemical-enzyme inhibition, induction or alteration of blood or tissue levels-dehydrogenase changes ;
3. Mutagenicity data: gene conversion and mitotic recombinationTEST system: yeast-Saccharomyces cerevisiae: 5ppm;
Micronucleus test IntraperitonealTEST system: rodent-mouse: 175mg/kg/ 24 hours.
4. It is slightly less toxic than o-dichlorobenzene and can be absorbed through the skin and mucous membranes. Can cause liver and kidney damage. The olfactory threshold concentration is 0.2mg/L (water quality).
5. Acute toxicity [16] LD50: 1062mg/kg (mouse vein); 1062mg/kg (mouse abdominal cavity)
6. Irritation No information available
7. Mutagenicity[17] Gene transformation and Mitotic recombination: Saccharomyces cerevisiae 5ppm. Micronucleus test: intraperitoneal administration of 175mg/kg (24h) to mice
8. Carcinogenicity [18] IARC Carcinogenicity Comments: Group 3. The available evidence cannot classify human carcinogenicity.
Ecological data
1. Ecotoxicity[19]
LC50: 21.8mg/L (24h) (bluegill, static); 12.7 mg/L (96h) (fathead minnow, static); 8.46mg/L (24h) (red perch, static)
2. Biodegradability[20]
Aerobic biodegradation (h): 673~4320
Anaerobic biodegradation (h): 2880~17280
3. Non-biodegradability[21]
Photolysis maximum light absorption wavelength range (nm): 216~278
Photooxidation half-life in air (h): 8.91~89.1
First-order hydrolysis half-life (h): >879a
4. Bioaccumulation [22 ] BCF: 60~230 (carp, exposure concentration 100μg/L, exposure time 8 weeks); 60~370 (carp, exposure concentration 10μg/L, exposure time 8 weeks); 420~740 (Rainbow trout, exposure time 119 days); 90 (Bluegill sunfish, exposure time 28 days)
5. Other harmful effects[23] The Substances are harmful to the environment, can cause pollution to water bodies and the atmosphere, and bioaccumulate in food chains important to humans, especially in aquatic organisms.
Molecular structure data
1. Molar refractive index: 36.04
2. Molar volume (cm3/mol): 113.3
3. Isotonic specific volume (90.2K ): 279.0
4. Surface tension (dyne/cm): 36.7
5. Polarizability (10-24cm3): 14.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 64.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. When chlorination reaction is carried out in the presence of ferric chloride or aluminum amalgam, 1,2,4-trichlorobenzene is mainly produced. In the presence of a catalyst, it is hydrolyzed at 550~850℃ to generate m-chlorophenol and resorcinol. Using copper oxide as a catalyst, it reacts with concentrated ammonia water at 150~200°C under pressure to generate m-phenylenediamine. When nitration or sulfonation occurs, 2,4-dichloronitrobenzene and 2,4-dichlorobenzenesulfonic acid are generated.
2. Stability[24] Stable
3. Incompatible substances[25] Strong oxidizer, aluminum
4. Conditions to avoid contact[26] Heating
5. Polymerization hazard[27] No polymerization
6. Decomposition products[28] Hydrogen chloride
Storage method
Storage Precautions[29] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, aluminum, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
The preparation methods are as follows.
Use chlorobenzene as raw material for further chlorination to obtain p-dichlorobenzene, o-dichlorobenzene and m-dichlorobenzene. The general separation method uses mixed dichlorobenzene for continuous distillation. Para- and meta-dichlorobenzene are distilled off the top of the tower. Para-dichlorobenzene is precipitated by freezing crystallization. The mother liquor is then rectified to obtain meta-dichlorobenzene. The column still is crude o-dichlorobenzene, and then flash distillate o-dichlorobenzene in the flash evaporation tower. At present, mixed dichlorobenzene adopts adsorption separation method, using molecular sieve as the adsorbent. The gas phase mixed dichlorobenzene enters the adsorption tower, which can selectively adsorb p-dichlorobenzene. The raffinate is meta- and ortho-dichlorobenzene. Distillation yields m-dichlorobenzene and o-dichlorobenzene respectively. The adsorption temperature is 180~200℃, and the adsorption pressure is normal pressure.
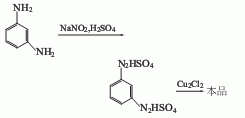
1. Meta-phenylenediamine diazo method: m-phenylenediamine is reconstituted in the presence of sodium nitrite and sulfuric acid.Nitridation, the diazotization temperature is between 0 and 5°C, and the diazo liquid is hydrolyzed in the presence of cuprous chloride to generate m-dichlorobenzene.
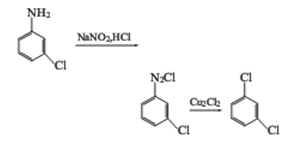
2. Meta-chloroaniline method: using meta-chlorine Aniline is used as raw material and is diazotized in the presence of sodium nitrite and hydrochloric acid. The diazo liquid is hydrolyzed in the presence of cuprous chloride to generate m-dichlorobenzene.
Among the above preparation methods, The most suitable and low-cost method for industrialization is the adsorption separation method of mixed dichlorobenzene. There are already domestic production facilities for production.
Purpose
1. Used in organic synthesis. Meta-dichlorobenzene and chloroacetyl chloride undergo Friedel-Crafts reaction to obtain 2,4,ω-trichloroacetophenone, which is used as an intermediate for the broad-spectrum antifungal drug miconazole. Chlorination reaction in the presence of ferric chloride or aluminum mercury mainly produces 1,2,4-trichlorobenzene. In the presence of a catalyst, it is hydrolyzed at 550-850°C to produce m-chlorophenol and resorcinol. Using copper oxide as a catalyst, it reacts with concentrated ammonia water at 150-200°C under pressure to generate m-phenylenediamine.
2. Used in dye manufacturing, organic synthesis intermediates and solvents. [30]

 微信扫一扫打赏
微信扫一扫打赏

