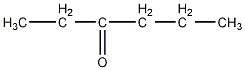
Structural formula
| Business number | 0603 |
|---|---|
| Molecular formula | C6H12O |
| Molecular weight | 100.16 |
| label |
Ethyl acetone, Ethyl propyl ketone, aliphatic compounds |
Numbering system
CAS number:589-38-8
MDL number:MFCD00009402
EINECS number:209-645-4
RTECS number:MP1575000
BRN number:1738025
PubChem number:24869932
Physical property data
1. Properties: transparent liquid. [1]
2. Melting point (℃): -55.4[2]
3. Boiling point (℃): 121.9~124[3]
4. Relative density (water=1): 0.81[4]
5. Relative vapor density (air = 1): 3.46[5]
6. Critical pressure (MPa): 3.32[6]
7. Octanol/water partition coefficient: 1.24[7]
8. Flash point (℃): 35 (OC) [8]
9. Ignition temperature (℃): 439[9]
10. Explosion upper limit (%): 8.0[10 ]
11. Lower explosion limit (%): 1.0[11]
12. Solubility: slightly soluble in water, soluble in Acetone is miscible in ethanol and ether. [12]
13. Refractive index (n20ºC): 1.4003
14. Refractive index (n25ºC): 1.3980
15 . Viscosity (mPa·s, 20ºC): 0.592
16. Critical density (g·cm-3): 0.265
17. Critical volume ( cm3·mol-1): 378
18. Critical compression factor: 0.259
19. Eccentricity factor: 0.396
20. Solubility parameter (J·cm-3)0.5: 17.898
21. van der Waals area ( cm2·mol-1): 8.540×109
22. van der Waals volume (cm3·mol-1): 69.730
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1) : -3797.78
24. Gas phase standard claimed heat (enthalpy) (kJ·mol-1) : -278.25
25. Gas phase standard entropy (J ·mol-1·K-1): 409.6
26. Gas phase standard free energy of formation (kJ·mol-1): -125.98
27. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3755.90
28. Liquid Phase standard claims heat (enthalpy) (kJ·mol-1): -320.13
29. Liquid phase standard entropy (J·mol-1 ·K-1): 305.3
30. Liquid phase standard free energy of formation (kJ·mol-1): -136.73
31. Liquid phase standard hot melt (J·mol-1·K-1): 217.1
Toxicological data
1. Acute toxicity[13] LD50: 3360mg/kg (rat oral); 3170mg/kg (rabbit dermal )
2. Irritation[14]
Rabbit transdermal: 500mg ( 24h), mild stimulation.
Rabbit eye: 500mg (24h), mild irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information yet
4. Other harmful effects[15] This substance may be harmful to the environment. Special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 29.87
2. Molar volume (cm3/mol): 124.6
3. Isotonic specific volume (90.2K ): 275.9
4. Surface tension (dyne/cm): 23.9
5. Polarizability (10-24cm3): 11.84
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 57.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances [17] Strong oxidizing agent, strong reducing agent, strong alkali
3. Polymerization hazard[18] No aggregation
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Preparation method:
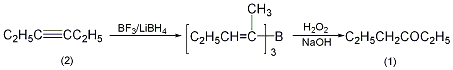
In a 0.5L reaction bottle, add 28.7g (0.35mol) of 3-hexyne (2) and 100mL (1.00mol/L) of sodium borohydride-diglyme solution. Add 0.135 mol of boron trifluoride-diethyl ether solution under stirring to generate an unsaturated organic boron compound. Slowly add 36mL of 30% hydrogen peroxide to keep the pH of the reaction solution at around 8. After the reaction, extract with diethyl ether, dry with anhydrous magnesium sulfate, evaporate the diethyl ether, and collect the fraction at 120-121°C or the fraction at 118°C/99.1kPa to obtain 23.8g of compound (1), yield 62%, nD 201.4004~1.4007. [21]
2. Preparation method:
![]()
Refer to the above preparation method of 3-pentanone (reference book 335), use n-butyric acid (2) 352g (4mol) and propionic acid 296 (6mol) to react , 214g of 3-hexanone (1) was obtained, bp 122~124℃, yield 53%. At the same time, 98g of 3-pentanone, bp 100-102°C and 66g 4-heptanone, bp 144-146°C were obtained as by-products. [22]
Purpose
1. Used as food spices. Mainly used for preparing wine and other fruit wine flavors and meat flavors.
2. Used as solvent. [20]

 微信扫一扫打赏
微信扫一扫打赏

