Overview[1]
2,4-Diiodoaniline can be used as a pharmaceutical synthesis intermediate. If 2,4-diiodoaniline is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if there is eye contact , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
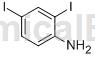
Structure
Preparation method[1]
The preparation of 2,4-diiodoaniline is as follows:
Method 1: In an inert atmosphere, add aniline (0.1g, 1.07mmol) and 1-butyl-3-methylpyridinium dichloroiodate (BMPDCI) (0.45g, 1.29mmol) into 10ml single neck In a round bottom flask. The reaction mixture was heated at 80°C for 1 hour. The reaction was monitored by TLC. After the reaction was complete (TLC), ethyl acetate (10 ml) was added followed by water (10 ml). The entire reaction mixture was extracted with ethyl acetate (3 x 10 ml). The combined ethyl acetate layers were washed with water, brine, and dried over anhydrous sodium sulfate. The separated combined organic layers were evaporated under vacuum to give crude product. The crude product was purified by silica gel column chromatography to obtain 0.315 g of pure 2,4-diiodoaniline (85% yield).
1HNMR (CDCl3δ/ppm): 7.89 (d, 1H, 7=2.02Hz, Ar-H), 7.39 (dd, 1H, 7=8.49, 7=2.0Hz, Ar-H), 6.53 (d, 7=8.34Hz, 1H, Ar-H), 4.12 (wide singlet, 2H, NH2).
Method 2: In an inert atmosphere, combine 10-iodo-aniline (0.1g, 0.45mmol) and 1-butyl-3-methylpyridinium dichloroiodate (BMPDCI) (0.19g, 0.547mmol) Add to 10ml single neck round bottom flask. The reaction mixture was heated at 80°C for 1 hour. The reaction was monitored by TLC. After the reaction was complete (TLC), ethyl acetate (10 ml) was added followed by water (10 ml). The entire reaction mixture was extracted with ethyl acetate (3 x 10 ml). The combined ethyl acetate layers were washed with water, brine, and dried over anhydrous sodium sulfate. The crude product was purified by silica gel column chromatography to obtain 0.15 g of pure 2,4-diiodoaniline (95% yield).
1HNMR (CDCl3δ/ppm): 7.89 (d, 1H, 7=2.02Hz, Ar-H), 7.39 (dd, 1H, 7=8.49, 7=2.0Hz, Ar-H), 6.53 (d, 7=8.34Hz, 1H, Ar-H), 4.12 (wide singlet, 2H, NH2).
Main reference materials
[1] WO2016113757 NOVEL RECYCLABLE IODINATING AGENT AND ITS APPLICATIONS

 微信扫一扫打赏
微信扫一扫打赏

