Overview[1]
(-)-1-[(4-Chlorophenyl)benzyl]piperazine can be used as a pharmaceutical synthesis intermediate, such as the synthesis of levocetirizine. Levocetirizine was launched in February 2001. It is a third-generation anti-allergic drug. Since it is the levorotatory body of cetirizine, its pharmacological effects are similar to that of cetirizine. It is mainly used to treat the respiratory system, skin and eyes. Allergic diseases such as allergic rhinoconjunctivitis, allergic skin diseases, allergic asthma, etc. have quick onset, strong and long-lasting effects, but avoid the sedation, drowsiness and other central nervous system effects of cetirizine. side effect. (-)-1-[(4-chlorophenyl)benzyl]piperazine is an important intermediate for the synthesis of levocetirizine. Some studies have disclosed the synthesis of (-)-1-[(4-chlorophenyl) ) A method of benzyl]piperazine, which comprises hydrolyzing 1-[(4-chlorophenyl)-phenylmethyl]piperazine-4-(4-methyl) with hydrobromic acid in the presence of 4-hydroxybenzoic acid. phenyl)sulfonylpiperazine. However, the purity and yield of levocetirizine during the industrialization process according to this method are not satisfactory.
Preparation method[1]
Step 1, preparation of chloroacetyl benzylamine
Add 5000g of purified water, 300g of benzylamine and 900g of sodium carbonate into the reaction vessel at one time, stir, control the temperature at 0-5°C, drop in 350g of chloroacetyl chloride, keep the reaction for 2 hours, filter with suction, 40-45°C , dried to obtain chloroacetyl benzylamine.
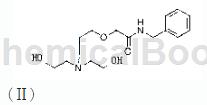
Step 2, preparation of intermediate II
Add 1750g of toluene and 50g of sodium hydroxide at one time into the reaction vessel, control the temperature at 20-25°C, add 100g of chloroacetylbenzylamine obtained in step 1 gradually, raise the temperature to 80-85°C, and react for 2 hours. After the temperature is lowered to 20-25°C, 50g of purified water is added, the pH is adjusted to 2-3 with dilute hydrochloric acid, and after filtration and drying at 40-45°C, the intermediate II of the present invention is obtained.
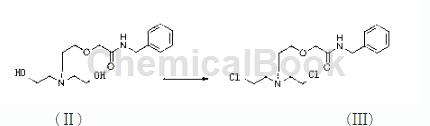
Step 3, preparation of intermediate III
Add 1500 ml of chloroform and 300 g of the intermediate II obtained in step 2 into the reaction vessel at one time, control the temperature at 20-25°C, add 5 ml of dimethylformamide dropwise, and then add 350 g of sulfoxide dichloride drop by drop, and keep the reaction 1 hour, reduce the pressure, control the temperature to 30-35°C, concentrate the chloroform and unreacted thionyl chloride until no distillate comes out, then add 2000kg of ice water and sodium hydroxide, and then extract twice with dichloromethane, each time The first dosage is 450g. Concentrate methylene chloride until no distillate comes out to obtain the intermediate III of the present invention.
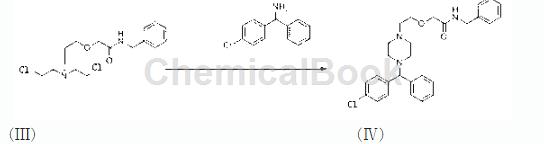
Step 4, preparation of intermediate IV
Add 200g of toluene, 100g of dipropylethylamine and 70g of intermediate III product into the reaction vessel at one time, stir, control the temperature to 105-110°C, and slowly add 35.2g of R-4-chlorodiphenylamine , reflux for 8 hours, concentrate the toluene under reduced pressure until no distillate comes out, add 100g of methanol, reflux for 1 hour, control the temperature under reduced pressure to T = 40-45°C, concentrate out the methanol and dipropylethylamine, and then control 100-105 ℃, and distilled under reduced pressure to obtain the intermediate IV of the present invention.
Step 5, preparation of (-)-1-[(4-chlorophenyl)benzyl]piperazine
Add 150g of water, 8g of potassium hydroxide, 65g of methanol, and 20g of the product obtained in step 4 into the reaction vessel. Stir and raise the temperature to about 70°C. Keep the reaction for 3 hours. Control the temperature at 40°C and concentrate under reduced pressure to recover the methanol. Add 5g of n-hexane and extract twice, and then extract twice with 40g of n-butanol. Combine the n-butanol, add activated carbon for decolorization, concentrate the n-butanol under reduced pressure, cool to 0-5°C, add 10g of n-hexane, Cool and crystallize under stirring, filter, dry at 0-5°C to obtain the title compound (-)-1-[(4-chlorophenyl)benzyl]piperazine.
Main reference materials
[1] CN103044355 Key intermediates for the synthesis of levocetirizine and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

