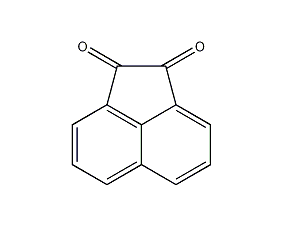
Structural formula
| Business number | 01T7 |
|---|---|
| Molecular formula | C12H6O2 |
| Molecular weight | 182.17 |
| label |
rylenedione, naphthoethylenedione, acenaphthene dioxide, Acenaphthyroquinone, Enaquinoline, Ethanaphthoquinone, naphthoquinoline, 1,2-Acenaphthylenedione, 1,2-Diketoacenaphthene |
Numbering system
CAS number:82-86-0
MDL number:MFCD00003805
EINECS number:201-441-3
RTECS number:AB1024500
BRN number:879172
PubChem number:24844961
Physical property data
1. Properties: Yellow needle-like crystals.
2. Density (g/mL, 16/2℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 261℃ (sublimation)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 3.73kPa ): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º) : Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined 19. Solubility: Soluble in alcohol, hot benzene and hot toluene, insoluble in water.
Toxicological data
Acute toxicity:
Rat LD50: 728 mg/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 51.91
2. Molar volume (cm3/mol): 127.8
3. Isotonic specific volume (90.2K ): 367.6
4. Surface tension (dyne/cm): 68.3
5. Polarizability (10-24cm3): 20.58
Compute chemical data
1. Reference values for calculation of hydrophobic parameters���XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Rotatable chemical bonds Number: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 267
10. Number of isotope atoms: 0
11. Determine the atoms Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is irritating to the eyes, respiratory system and skin. Wear appropriate protective clothing if used in large quantities. If it comes into contact with eyes, rinse immediately with plenty of water and seek medical advice.
Storage method
Store sealed and dry.
Synthesis method
Obtained from the oxidation of acenaphthene with sodium dichromate: In a stainless steel reactor with a cooling jacket, add acenaphthene; glacial acetic acid and ceric acetate, stir, add a measured amount of sodium dichromate dihydrate within 2 hours, and keep the temperature at 40℃. Stirring was then continued at room temperature for 8 h. Dilute with cold water, filter, and wash with water until there is no acidity. Boil the solid and 10% sodium carbonate solution on a steam bath for 30 minutes, filter and wash. Then extract the solid with sodium bisulfite (4% solution) at 80°C for 30 minutes, then add filter aid diatomaceous earth and activated carbon, and then filter. Repeat the extraction once, combine the filtrate and acidify it with concentrated hydrochloric acid at 80°C with constant stirring until the Congo red test paper turns blue, and continue to keep stirring at 80°C for 1 hour. Acenaphthyroquinone is separated out as bright yellow crystals, filtered, and washed with water to remove the acidity, to obtain acenaphthyroquinone with a yield of 38%-60%. Melting point 256-260℃. Recrystallize with o-dichlorobenzene, and after washing the crystal with methanol, the melting point can reach 256-260°C. It can also be obtained by using acenaphthene as a raw material, reacting with sodium nitrite in a hydrochloric acid solution to form acenaphthene ketone oxime, and then reacting with formaldehyde in sulfuric acid.
Purpose
1. Acenaphthyroquinone is an intermediate of dyes and pesticides. Dye synthesizers. Pesticides.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/35-1.jpgextended-reading:https://www.bdmaee.net/jeffcat-zf-26-catalyst-cas3033-62-3-huntsman/extended-reading:https://www.cyclohexylamine.net/dabco-xd-104-dabco-tertiary-amine-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/37-2.jpgextended-reading:https://www.cyclohexylamine.net/n-dimethylaminopropyldiisopropanolamine-cas-63469-23-8/extended-reading:https://www.bdmaee.net/dabco-dc2-delayed-catalyst-dabco-dc2-delayed-catalyst-dabco-dc2/extended-reading:https://www.newtopchem.com/archives/category/products/page/43extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/53.jpgextended-reading:https://www.newtopchem.com/archives/45194extended-reading:https://www.newtopchem.com/archives/44507

 微信扫一扫打赏
微信扫一扫打赏

