Background[1]
Ortho-Chlorob ℃ nzoic acid is a colorless needle or monoclinic crystal with a melting point of 142°C, sublimation when heated, and a relative density of 1.544; easily soluble in alcohol and ether, soluble in 900 parts of cold water, and more In hot water, insoluble in toluene. O-bromobenzoic acid is an important fine organic chemical product. It is an important intermediate and analytical reagent in the fields of dyes, pesticides, medicines, etc. It can be used to prepare bromopromazine, anti-inflammatory spirit, mebendazole, dibromethacin, and gram. Triconazole and other drugs are standard reagents for alkali and iodometric methods. They are used as preservatives for adhesives and paints. They can also be used as raw materials for organic synthesis, dyes and color films. Their market prospects are relatively broad.
Preparation
As an important fine organic chemical product, o-bromobenzoic acid has many domestic and foreign manufacturers; according to literature reports, its production process mainly includes three methods: diazotization, oxidation, and bromination.
1. Diazotization method
This process uses phthalic anhydride as raw material. First, it undergoes amidation reaction with sodium hydroxide and ammonium hydroxide to generate sodium anthranilic acid. After degradation reaction of sodium hypobromite, sodium anthranilic acid is generated, which is neutralized and Refined anthranilic acid is obtained; then diazotization reaction is carried out with sodium sulfite and hydrochloric acid. Under the catalysis of cuprous salt, the obtained diazonium salt replaces the diazo group with a bromo group to produce Sandmeyer (sandm℃y-℃ r) Conversion reaction to obtain crude o-bromobenzoic acid, which is then refined to obtain the finished product.
The process is as follows:
1) Amidation: Put ammonia water and ice into the reactor, stir, wait until the temperature drops to 0℃, add phthalic anhydride, naturally raise the temperature to 20℃, add liquid sodium hydroxide, and keep it at 60~70℃ for 1 hour; 65~70 ℃ to discharge ammonia for about 2 hours. When the pH value of the gas in the kettle is measured to be 8.5~9.0, the temperature is lowered to 30℃ to obtain sodium o-carbamidobenzoate solution.
2) Degradation: Cool the sodium o-formyl benzoate solution to 30°C, add liquid sodium hydroxide; stir and add ice, cool to 0°C, quickly add the calculated amount of sodium hypobromite, naturally warm to 40°C, add Sodium bisulfite, add ice to keep below 0°C to obtain sodium anthranilate solution.
3) Neutralization and refining. Cool the above reaction solution to below 25°C and stir, adjust the pH to 3.5~4 with hydrochloric acid, cool to 25~30°C, filter, wash with 30~40°C water, and spin dry in a centrifuge. , dry to obtain crude anthranilic acid. Dissolve with 5% hydrochloric acid, press filter, adjust the pH of the filtrate to 3.5~4 with ammonia water, filter and dry to obtain the fine product.
4) Diazotization and transformation Add the refined anthranilic acid, hydrochloric acid and water to the reactor in sequence, stir and dissolve, cool, and add sodium nitrite solution dropwise at 0~5°C. After the dropwise addition is completed, continue the stirring reaction for 0.5h to obtain a diazo liquid; add it to the cuprous bromide hydrochloric acid solution in batches, leave it for more than 2 hours after the addition, then filter, wash with water, and dry to obtain the o-bromobenzoic acid product.
2. Oxidation method
The air-liquid phase oxidation of hydrocarbons can directly produce a series of products such as organic hydrogen peroxide, alcohols, ketones, and hydroxy acids in industry. The air liquid phase oxidation reaction has the advantages of not consuming expensive chemical oxidants, cheap catalysts, low reaction temperature, good selectivity, and controllable oxidation depth.
3. Bromination method
As mentioned above, there are many process routes for the synthesis of ortho-bromobenzoic acid. The bromination method uses ortho-Chlorotoluene as the raw material and is first brominated into ortho-chlorotrichlorotoluene. It is then hydrolyzed to o-bromobenzoic acid. The current industrial production of o-bromotoluene is made by using o-bromotoluene as raw material and passing bromine gas in the presence of phosphorus tribromide catalyst
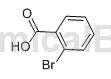
O-bromobenzoic acid
Apply[3]
O-chlorobenzoic acid is an important intermediate with a wide range of uses in the pesticide, pharmaceutical and dye industries. In pesticides, it is mainly used to synthesize insecticides (Threonil No. 1) and fungicides. In medicine, it is mainly used It is used to synthesize the antipsychotic drug perphenazine, the adrenomimetic drugs Zengrubenxin and Chuantong, the antifungal drugs clotrimazole, chlorpromazine, ketamine and diclofenac, etc. It is the standard reagent for the alkali method and the iodine method. It is used as a preservative for adhesives and paints, and can also be used as raw materials for organic synthesis, dyes and color films. Its market prospects are broad. As an important fine organic chemical product, o-chlorobenzoic acid has been extensively studied in recent years.
Using o-bromobenzoic acid and p-methoxyaniline as raw materials, potassium carbonate andCopper powder is used as a catalyst, isoamyl alcohol or n-amyl alcohol is used as a solvent, and 2-methoxy-9-acridine (p-methoxyphenyl)-1,2,3-triazole is obtained through Ullmann reaction:
In a 250mL three-neck flask, add 5.20g (26mmol) of o-bromobenzoic acid, 4.19g (34mmol) of p-methoxyaniline, 7.5g (36.2mmol) of potassium carbonate and 0.3g (4.7mmol) of copper powder. Then add 30 mL of isoamyl alcohol as the solvent, stir and react under reflux at 140°C for 2 hours. After the reaction, evaporate the solvent under reduced pressure. Add 600 mL of water to the residue, stir for 20 minutes at 80°C, filter while hot, wash the filter cake with water, and combine the water. layer, the aqueous layer was acidified to pH=2 with concentrated hydrochloric acid, and a large amount of light green precipitate precipitated, which was suction filtered, and the obtained solid was recrystallized with chloroform to obtain compound 2-methoxy-9-acridine (p-methoxyphenyl)-1 ,2,3-triazole, yield 79%;
Main reference materials
[1] Li Meichao, Bao Dandan, & Ma Chun’an. (2010). In-situ infrared spectroscopy study of electrochemical debromination reaction of o-bromobenzoic acid. Journal of Chemistry in Colleges and Universities, 32(1), 129-133.
[2] Zhao Yongliang, Zhao Fengying, Lian Xishan, & Chang Linghua. (1997). Synthesis and characterization of o-bromobenzoic acid-o-phenanthroline lanthanum and samarium ternary solid-state complexes. Journal of Inner Mongolia University: Nature Science Edition(1), 62-64.
[3] Zhao Yongliang, Lian Xishan, & Zhao Fengying. (1993). Spectral characteristics of o-bromobenzoic acid rare earth complexes. Journal of Inner Mongolia University (Nature Edition) (5), 510-513.

 微信扫一扫打赏
微信扫一扫打赏

