Background and overview[1]
3-Methylsulfonylbenzaldehyde can be used as a pharmaceutical synthesis intermediate. If 3-methylsulfonylbenzaldehyde is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if there is eye contact , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
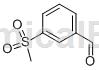
Structure
Preparation[1]
The preparation of 3-methylsulfonylbenzaldehyde is as follows:
![]()
1) A mixture of 3-methylsulfonylbenzoic acid (1.0 mmol), methyl iodide (1.2 mmol) and potassium carbonate (2 mmol) in 20 ml acetone was heated at 50°C for 3 hours. The solvent from the reaction mixture was removed under reduced pressure. The residue was partitioned between EtOAc and water. The aqueous solution was extracted with EtOAc, and the combined organic solutions were washed with brine, dried over Na2SO4, filtered, and concentrated. The crude product as a white solid (98% yield) was used in the next step without purification.
2) Slowly add LiAlH (1.1mmoL) to the solution of the compound prepared above (1mmoL) in 4mLTHF at 0°C. The reaction mixture was warmed to room temperature and stirred for 1 hour. Under vigorous stirring, the reaction mixture was added successively to water, 15% NaOH aqueous solution and water. Filtration and evaporation of the filtrate gave crude product 8.6 (yield 92%). No purification is required. 1HNMR (400MHz, CDCl3) δ. 3.05 (s, 3H), 4.75 (s, 2H), 7.53 (t, J=7.58Hz, 1H), 7.62 (d, J=7.34Hz, 1H), 7.81 (d, J=7.83Hz), 1H) ,7.93 (s5IH).
3) Add solid tetrapropyl ammonium perruthenate (“TPAP”, 0.05mmol) to compound 8.6 (1mmoL), 4-methylmorpholine N-oxide (“NMO”; 1.5mmoL) in one go and powdered 4A molecular sieve in a stirred mixture. (Equal weight of NMO in 5 mL DCM at room temperature under N2). The reaction mixture was stirred at room temperature for 1 h and then filtered through a short pad of silica gel, eluting with a mixture of DCM and AcOEt (1:1). The filtrate was concentrated, and the residue was purified by chromatography (SiO2, AcOEt/hexane 2:1) to obtain compound 3-methylsulfonylbenzaldehyde (yield 72%). 1HNMR (400MHz, CDCl3) δ3.14 (s, 3H), 7.81 (t, J=7.58Hz, 1H), 8.21 (t, J=9.05Hz, 2H), 8.46 (s, 1H), 10.12 (s, lH)ppm.
Main reference materials
[1] WO2005044817 MODULATORS OF CELLULAR ADHESION

 微信扫一扫打赏
微信扫一扫打赏

