Background and overview[1]
4-(3-Bromophenyl)piperidine hydrochloride can be used as a pharmaceutical synthesis intermediate. If 4-(3-bromophenyl)piperidine hydrochloride is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse skin thoroughly with soap and water. If discomfort occurs , seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 4-(3-bromophenyl)piperidine hydrochloride is as follows: 4-(3-bromo-phenyl)-piperidine (1.0g, 3.6mmol) is added to hydrochloride, and the reaction product is isolated. 4-(3-Bromophenyl)piperidine hydrochloride, a white solid (0.43 g, 41%), was used without further purification. 1HNMR (400MHz, (D3C)2SO, δ, ppm): 10.52 (s, 1H), 7.47-7.41 (m, 2H), 7.37-7.21 (m, 2H), 3.52-3.41 (m, 2H), 3.10- 2.95 (m, 2H), 2.85-2.78 (m, 1H), 2.75 (s, 3H), 2.15-2.78 (m, 4H).
Apply[1]
4-(3-Bromophenyl)piperidine hydrochloride can be used as a pharmaceutical synthesis intermediate. As prepared:
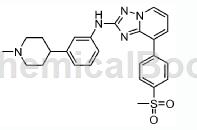
Composed of 8-(4-methanesulfonyl-phenyl)-[1,2,4]triazolo[1,5-a]pyridin-2-ylamine (75.0mg, 0.260mmol) and 4- (3-Bromophenyl)piperidine hydrochloride was used as the reactant, and 2,2′-bis-dicyclohexylphosphoryl-biphenyl (30.0 mg, 0.0549mmol) was used as the ligand. Hydrochloride (90.0 mg ,0.310mmol). The title compound was isolated as a brown solid (0.031 g, 26%). MP=208-210℃. 1HNMR (400MHz, CDCl3, δ, ppm): 8.51 (d, J=6.5Hz, 1H), 8.08 (d, J=8.3Hz, 2H), 8.10 (d, J=7.3Hz, 2H), 7.66 (d , J=6.6Hz, 1H), 7.55 (s, 1H), 7.38 (d, J=8.7Hz, 1H), 7.31-7.25 (m, 1H), 7.02 (t, J=7.4Hz, 1H), 6.91 -6.85 (m, 2H), 3.10 (s, 3H), 3.01 (d, J=10.9Hz, 2H), 2.57-2.45 (m, IH), 2.35 (S, 3H), 2.11-2.03 (m, 2H ), 1.92-1.83 (m, 4H). MS=462(MH)+.
Main reference materials
[1] (WO2010141796)PREPARATIONANDUSESOF1,2,4-TRIAZOLO[1,5a]PYRIDINEDERIVATIVES

 微信扫一扫打赏
微信扫一扫打赏

