Overview
Benzocyclobutene (BCB) monomer has the thermodynamic stability of aromatic compounds and the kinetic reactivity of strained rings[1 ]. After heating to 200°C, the four-membered ring opens and forms a highly reactive polymerizable intermediate. This polymerizable intermediate can not only undergo Diels-Alder addition reaction with dienophilic monomers, but also react with each other to form polymers[2] . As a new type of reactive resin, benzocyclobutene resin can form both thermoplastic resin and thermosetting resin[3], It has excellent thermal stability, molding processability, low dielectric constant (K), low water absorption and low thermal expansion coefficient. Based on these excellent properties, benzocyclobutene resin has been widely used in electronics, microelectronics industry and other fields [4-5], Specific applications include enameled wire varnish, wafer-level packaging materials, liquid crystal displays, interlayer dielectrics in semiconductor packaging, very large-scale integrated circuits and fiber-reinforced composite materials. Benzocyclobutene resin has become a current research hotspot in the field of dielectric materials.
With the development of very large scale integrated circuits and multi-chip components, the requirements for interlayer dielectric materials are becoming higher and higher. The materials must not only have excellent dielectric properties, but also have excellent thermal stability, Water resistance, etc. A single BCB resin material can no longer meet the requirements of these applications in terms of performance, so it is necessary to introduce other groups to improve the performance of BCB resin. For example, the introduction of silicon- and fluorine-containing groups can improve the thermal stability and dielectric properties of the resin. wait.
Ring-opening polymerization mechanism
Due to the multifunctional reactivity, superior electronic and thermal properties of benzocyclobutene monomers, benzocyclobutene functional polymers are regarded as a new generation of high-performance materials. Benzocyclobutene monomer has a four-membered ring structure maintained by stress. Under heating (more than 200°C), the four-membered ring opens, and BCB monomer forms a highly active conjugated diene intermediate (o-quine). Nozodimethane). If the BCB four-membered ring contains different substituent groups, the ring-opening temperature of the four-membered ring will be reduced to varying degrees [1]. In the presence of dienophilic monomers, the intermediate formed after benzocyclobutene ring-opening preferentially undergoes Diels-Alder addition reaction with dienophilic monomers. If there is no dienophilic monomer, the intermediate will Intermediates can also react with each other to form spirocyclic intermediates, which can then undergo a series of rearrangements to obtain polymers and oligomers. The ring-opening polymerization of benzocyclobutene monomer does not require the addition of catalysts and initiators, and no small molecule by-products are produced during the reaction. The reaction mechanism of BCB participating in ring-opening polymerization is shown in the figure.
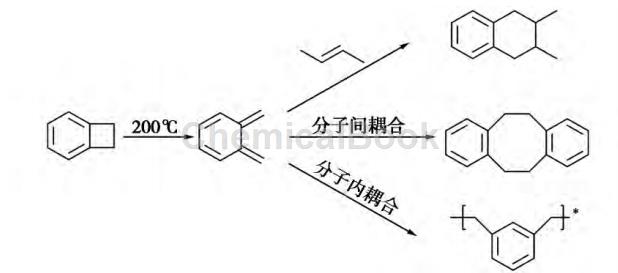
Benzocyclobutene resin
As a new type of high-performance thermosetting material, benzocyclobutene resin does not require the addition of any catalyst during the curing process and does not produce volatile small molecules and other by-products. Benzocyclobutene cured resin has excellent film-forming properties, thermal stability, adhesive properties and dielectric properties, and is a new generation of high-performance dielectric materials. With the development of very large scale integrated circuits and multi-chip components, the requirements for interlayer dielectric materials are getting higher and higher. The material properties of single benzocyclobutene resin can no longer meet the needs of applications. Here is an introduction to BCB resin Silicones, fluorine elements, low-polarity bulky and other polymerizable monomers are introduced to improve the material properties of the resin. Generally speaking, the dielectric constant and loss factor of polymers are inversely proportional to the molar polarization of the molecular structure and directly proportional to the density.
(1) Silicone benzocyclobutene resin. In the past few decades, people have devoted themselves to the research of silicone polymers, such as polysiloxane, polycarbosiloxane, polycarbosilane, etc. Among these silicone polymers, polysiloxanes have been widely used due to their excellent flexibility, biocompatibility, weather resistance, oxidation resistance, hydrophobicity and low glass transition temperature (Tg). At the same time, silica-based material is a widely used low-dielectric material, so introducing a siloxane structure into the resin can improve the hydrophobicity, weather resistance and dielectric properties of the resin.
(2) Fluorine-containing benzocyclobutene resin. Benzocyclobutene-based polymers have been widely used in the field of microelectronics, but in many cases, the K value of benzocyclobutene resin is greater than 2.7, which is not suitable for applications in high-frequency printed circuit boards and very large-scale integrated circuits. Not satisfying. It has been noticed that the introduction of fluorine groups into the main chain or side chains of polymers can effectively improve their dielectric properties.
(3) Benzocyclobutene resin containing low polarity and large volume functional groups. In order to improve the performance of materials, low-polar bulky functional groups are often introduced into the polymer structure. Since the chemical bonds in the functional groups have low polarizability and the functional groups contain large-volume structures, which can increase the free volume of the polymer, the introduction of low-polar large-volume rigid functional groups can improve the dielectric and thermal stability of the resin.
(4) Benzocyclobutene resin containing other polymerizable monomers. At 200°C, benzocyclobutene can ring open to form highly active conjugated diene monomers. This monomer can react with dienophilic monomers.�Alder addition reaction can also react with each other to form polymers and oligomers. No catalysts and initiators are required during the polymerization process and no small molecule by-products are released. In view of the unique ring-opening mechanism of BCB, scholars have introduced BCB groups into other materials to improve the dielectric properties, thermal stability and other properties of the materials. Several materials are introduced below, such as benzoxazine (BOZ) resin, polym-phenylene (PMPs), aromatic polycarbonate and natural resource anise oil.
References
[1] Huang Farong. Benzocyclobutene polymer materials[J]. Journal of Aeronautical Materials, 1998, 18(2): 53-62.
[2] Yang Military Academy. Synthesis of benzocyclobutene monomer and study on its polymer properties[D]. Chengdu: Sichuan University, 2005.
[3] Wang Jing, Zhang Fuxin, Shen Xuening, etc. Benzocyclobutene and its materials[J]. Fiberglass/Composite Materials, 2002, (2): 44-50
[4]Hu Zuowen, Chen Mengxue. Synthesis and performance study of new benzocyclobutene resin[J]. New Chemical Materials, 2008, 36(11): 44-45.
[5]Xu Tingting, Liang Guozheng, Lu Tingli, et al. Synthesis of benzocyclobutene compounds[J]. Polymer Bulletin, 2006, (11): 69-77.

 微信扫一扫打赏
微信扫一扫打赏

