Background and overview[1][2]
Phenolic compounds are important secondary metabolites produced by plants such as ginkgo, onion, bitter bark, Scutellaria barbata, and rhubarb. They often show antibacterial, insecticidal, herbicidal and other biological activities, and are suitable for the development of botanical pesticides. Lead compounds are provided. Several carbonyl-containing phenolic compounds (i.e., hydroxyaromatic ketones) were isolated from Scutellaria barbata and found to have a certain bactericidal effect. It is planned to modify these compounds through biomimetic synthesis. Due to the large electronegativity and small radius of the fluorine atom, the C-F bond energy formed is much greater than the C-H bond energy, which makes the organic fluorine compound very stable. When the fluorine-containing group is introduced into the active compound, due to The electronic effects, blocking effects, pseudo-effects, and permeability effects of fluorine-containing substituents work together to produce complex physiological activities.
The creation of fluorine-containing drugs has become an important direction in the development of new medicines and new pesticides. O-fluorophenol is an important pharmaceutical intermediate. There are many methods for preparing o-fluorophenol. According to the raw material route, there are mainly low-temperature fluorination of phenol, o-fluorobromobenzene hydrolysis, and o-fluoroaniline diazotization hydrolysis. Among them, the diazotization hydrolysis method of o-fluoroaniline is a commonly used method. It uses o-fluoroaniline as raw material, diazotizes it with sodium nitrite, and then hydrolyzes it in dilute acid at high temperature to obtain o-fluoroaniline. The operation is simple and the reaction conditions are mild. . However, it is easy to produce waste acid containing sodium salt, and organic solvents or salt solutions are often added during the hydrolysis process, which effectively increases the cost.
Apply[2-5]
O-Fluorophenol is mainly used as an intermediate for the synthesis of new fluorinated antibacterial and anti-inflammatory drugs and insecticides, paraffinicides, herbicides and liquid crystal materials. Examples of its application are as follows:
1. Preparation of 2-chloro-6-fluorophenol 2-chloro-6-fluorophenol is an important fluorine-containing fine chemical,
It is also an important pharmaceutical and pesticide intermediate. Specifically, it includes the following steps: (1) Add o-fluorophenol to the reactor, slowly add sodium hypochlorite aqueous solution dropwise into the reactor, the molar ratio of sodium hypochlorite to o-fluorophenol is (1.0~2.0):1, and heat the reactor to 0~77 ℃, heat preservation reaction to generate a mixture of 2-chloro-6-fluorophenol; (2) After the reaction is completed, add dilute hydrochloric acid to the reactor, adjust the pH to 5~6.5, and let it stand to separate the organic phase; (3) Separate the step (2) The organic phase was purified and dried to obtain 2-chloro-6-fluorophenol. The above preparation method is simple to operate and low in cost, and can be produced in large quantities on an industrial scale.
2. Preparation of 3-fluoro-4-hydroxy-1-phenylbutanone.
Use o-fluorophenol and n-butyric acid as raw materials, use anhydrous zinc chloride as the catalyst, react at 80-200°C, treat the reaction mixture with hydrochloric acid, and extract with an organic solvent to obtain the target compound from the organic phase. This compound has a high inhibitory effect on plant pathogenic bacteria such as apple rot, citrus anthracnose, cabbage gray mold, cotton wilt, and wheat total erosion, and has a good inhibitory effect on weeds such as barnyard grass.
3. The method described for preparing 1,2-dialkoxy-3-fluorobenzene uses 2-fluorophenol as raw material,
After sulfonation, halogenation, and deprotection, the intermediate 2-fluoro-6-halogenated phenol is obtained; then the intermediate 2-fluoro-6-halogenated phenol is etherified, introduced into the hydroxyl group, and then etherified, etc. A multi-step reaction is performed to prepare 1,2-dialkoxy-3-fluorobenzene; or the intermediate 2-fluoro-6-halogenated phenol is hydroxylated and etherified to prepare 1,2-dialkoxy Base-3-fluorobenzene. This method has low cost and high yield, and is suitable for industrial production.
4. Preparation of 2-fluoro-4-nitrophenol.
The following steps are adopted: a) Nitrosation reaction: 2-fluorophenol is used as raw material, and nitrosation is performed in the presence of dilute hydrochloric acid to generate 2-fluoro-4-nitrosophenol. The concentration of dilute hydrochloric acid is 15~20%, preferably 15%; the nitrosation reagent is an alkali metal nitrite salt or nitrite ester, preferably sodium nitrite; the nitrosation reaction temperature is -5~5°C, preferably 0°C; b) oxidation Reaction: oxidize 2-fluoro-4-nitrosophenol with dilute nitric acid to prepare 2-fluoro-4-nitrophenol. The concentration of dilute nitric acid used is 15~55%, preferably 30%. The invention has the beneficial effects that the preparation process is simple, only a very small amount of isomers are produced during nitrosation, the yield is as high as 90%, the purification is also easy, the purity can reach more than 99.5%, and the production cost is reduced.
Preparation [1,6]
There are many synthetic methods for preparing o-fluorophenol. According to the raw material route, there are mainly low-temperature fluorination of phenol, o-fluorobromobenzene hydrolysis, o-fluoroaniline diazotization hydrolysis, etc.
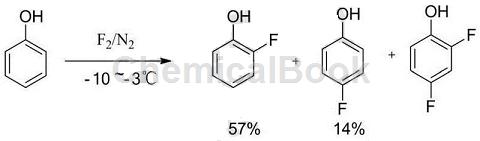
1) Dissolve phenol in an appropriate amount of solvent, cool it to -10~-3°C, and then pass in fluorine gas diluted with nitrogen to react to obtain o-fluorophenol and p-fluorophenol. The ratio is 57:14. This process route not only requires the use of highly dangerous fluorine gas, but also the two isomers of o-fluorophenol and p-fluorophenol in the product are difficult to separate, and tar-like by-products will also be generated. . The synthetic equation is shown in equation:
2) Use o-fluorobenzene as raw material, add it with barium hydroxide, water, copper and a small amount of catalyst into an autoclave, and react at high temperature for a period of time to obtain o-fluorophenol. This method has a good yield. However, the price of o-fluorobenzene is almost the same as that of o-fluorophenol, so this method has almost no economic benefits. See equation:
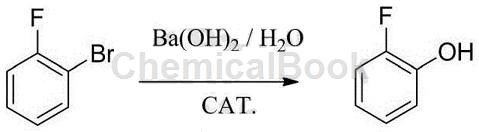
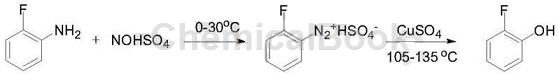
3) Using o-fluoroaniline as raw material, it is diazotized with sodium nitrite, and then hydrolyzed at high temperature in dilute acid to obtain o-fluorophenol. The advantages of this method are simple operation, mild reaction conditions, and convenient sources of raw materials. Includes the following steps:
1) Add water into the reactor, and then slowly add 98% sulfuric acid into the reactor, stirring while adding. After the dropwise addition is completed, the temperature of the liquid in the reactor is 50-60°C; among them, water, The mass ratio of sulfuric acid is 1:3.5;
2) Quickly add o-fluoroaniline into the reactor while stirring. After the dropwise addition is completed, cool to 5-10°C. Add 10% nitrosyl sulfuric acid solution into the reactor and stir for 1 hour; finally Add urea, stir for 2 hours at 10-15°C, and store the mixture in a refrigerator; the mass ratio of o-fluoroaniline, sulfuric acid, and nitrosyl sulfate solution is 1:1:1; nitrosyl sulfate solution , the mass ratio of urea is 3:0.01;
3) After preheating the pipeline reactor with steam, adjust the steam pressure to 0.5MPa. The receiving bottle in the pipeline reactor is cooled by frozen brine. A condenser tube is installed at the tail gas outlet and frozen brine is used for cooling. The above system is The obtained mixed liquid is added to the tubular reactor, and a peristaltic pump is used to adjust the addition rate of the mixed liquid to 300g/h, and a thermal decomposition reaction is performed at 100-110°C to obtain the reaction liquid;
4) Filter the above reaction solution, add toluene for extraction 4 times, and combine the extracted organic phases; distill the organic phase under reduced pressure at 60°C until no liquid comes out, and then slowly raise the temperature to 120°C for distillation A light yellow liquid is produced, and the remaining product is cooled and solidified to obtain the product o-fluorophenol.
Main reference materials
[1] CN201810413047.9 An efficient synthesis method of o-fluorophenol
[2] CN201410021599.7 Preparation method and agricultural biological activity of compound 3-fluoro-4-hydroxy-1-phenylbutanone
[3] CN201310382592.3 Preparation method of 2-chloro-6-fluorophenol
[4] Preparation method of CN200610040653.82-fluoro-4-nitrophenol
[5] CN200510008983.4 A method for preparing 1,2-dialkoxy-3-fluorobenzene via the intermediate 2-fluoro-6-halogenated phenol
[6] CN201510856044.9 A method for tubular continuous production of o-fluorophenol
.9 A method for tubular continuous production of o-fluorophenol

 微信扫一扫打赏
微信扫一扫打赏

