Background and overview[1][2]
2,4-Dimethylphenol, needle-like crystal. The melting point is 27-28℃, the boiling point is 211.0℃, the relative density is 0.9650 (20/4℃), and the refractive index is 1.5420 (14℃). Miscible with alcohol and ether, slightly soluble in water.
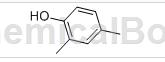
Preparation[1]
CN201810102442.5 provides a separation process for extracting 2,4-dimethylphenol from crude phenol.
The specific steps are as follows:
(1) Distillation and Separation 1
Using crude phenol as raw material, using preheating method and continuous distillation method, m-cresol and p-cresol are separated from the top of the distillation tower. The components in the tower kettle are 2,4-dimethylphenol, 2,5-dimethylphenol and high boiling substances enter the second step of distillation and separation;
(2) Distillation and Separation II
Continuously pump the still material of the distillation and separation tower into the second distillation and separation tower, using the preheating method. Through the continuous distillation method, the feeding method in the tower is used, and 2,4-dimethyl is continuously extracted from the top of the tower. Phenol and 2,5-dimethylphenol, used in the three steps of alkylation synthesis;
(3) Alkylation synthesis of tri
Using 2,4-dimethylphenol and 2,5-dimethylphenol extracted from the top of the second distillation separation tower as raw materials, using solid super acid ZSM-5 as catalyst, using a solid bed, and the dosage is 5% , pass in excess isobutylene, reaction temperature is 60~65℃, reaction pressure is 0.1MPa, and generate mono-tert-2,4-dimethylphenol and mono-tert-butyl 2,5-dimethylphenol;
(4) Distillation and separation four
Mono-tert-butyl 2,4-dimethylphenol and mono-tert-butyl 2,5-dimethylphenol are preheated and separated by continuous distillation, and mono-tert-butyl 2,4 is extracted from the top of the tower. – Dimethylphenol enters the fifth step of dealenization separation, and the mono-tert-butyl 2,5-dimethylphenol extracted from the four-column reactor is distilled and separated, and enters the sixth step of dealenization separation;
(5) De-ene separation five
Using mono-tert-butyl 2,4-dimethylphenol extracted from the top of the fourth tower of distillation and separation as raw material, add 0.1% sulfuric acid at a temperature of 130 to 150°C to remove isobutylene and return to the third step of alkylation synthesis. Use, remove 2,4-dimethylphenol, purity ≥ 99.9%;
The present invention uses crude phenol, a simple and cheap coal chemical waste material, as raw material, uses distillation, synthesis, and crystallization methods to isolate 2,4-dimethylphenol with higher purity, and uses this method to prepare 2, 4-Dimethylphenol has the characteristics of high purity, high yield, low cost, and is suitable for large-scale production.
Application [2-4]
1. Used to synthesize an asymmetric hindered phenol antioxidant
Hindered phenolic antioxidant is a widely used anti-aging agent for plastics, rubber, and polymer materials. It can effectively protect plastics, rubber, and polymer materials from the aging damage of oxygen in the environment, thereby extending the life of the material. service life. CN201310347375.0 provides an asymmetric hindered phenol antioxidant and a synthesis method thereof. Its chemical name is 2,4-dimethyl-6-styrenephenol. The synthesis method includes the following steps:
1) Put 155-165g liquid 2,4-dimethylphenol and 15-20ml boron trifluoride ether solution under normal pressure into a reaction equipped with a reflux condenser, a constant pressure dropping funnel and a stirrer. Mix in the reactor, stir evenly at a speed of 600-650r/min and heat it up to 45-50°C. Add 300-320g liquid styrene dropwise into the reactor under the protection of nitrogen and stir evenly at a speed of 670-690r/min. Add dropwise for 2 to 2.5 hours, and the dropwise addition ends;
2) After 1.5 to 2 hours of insulation reaction, turn off the nitrogen protection, cool to 25 to 30°C, add pure dichloromethane solvent for dilution for 1 to 1.5 hours;
3) Then add saturated sodium bicarbonate aqueous solution and wash with water for 1 to 1.5 hours, then wash with 3*300ml water three times, and wash until neutral to obtain water and oil separation;
4) Static layering, separate all the water and release the oil layer, add anhydrous sodium sulfate to the oil layer for dehydration for 12 hours;
5) Use a Buchner funnel, filter bottle, hose, air pump and filter paper to filter. Use a hose to connect the Buchner funnel and the filter bottle. Check whether the connection is tight and whether the connection port of the air pump is leaking. Before pumping The filter bottle is equipped with a single-hole plug, and the Buchner funnel is installed in the plug hole. The bevel at the lower end of the Buchner funnel tube faces the air extraction nozzle. Trim the filter paper so that it is slightly smaller than the cloth funnel and covers the cloth funnel. Add distilled water dropwise. The filter paper and funnel are tightly connected; add a small amount of water to the filter paper, gently open the faucet, and suck out some of the air in the filter bottle.��Put the filter paper close to the bottom of the funnel, then turn on the air pump switch, extract for 15-20 minutes until the vacuum degree is 15-20mmHg, and separate the filtrate;
6) Distill the above filtrate under normal pressure to 55°C to remove methylene chloride and collect all the methylene chloride, then reduce the pressure to a vacuum degree of 10-15mmHg and distill until the internal temperature reaches 70°C, then remove unreacted raw materials. , cool, and the light yellow oil layer liquid poured out is the final product 2,4-dimethyl-6-styrenephenol.
2. Used to prepare 2,4-dimethyl-6-(1-methyl-pentadecyl)phenol
CN201410813720 provides a method for preparing the hindered phenol antioxidant 2,4-dimethyl-6-(1-methyl-pentadecyl)phenol. The specific technical scheme is as follows: the hindered phenol antioxidant of the present invention The preparation method of agent 2,4-dimethyl-6-(1-methyl-pentadecyl)phenol is to feed 2,4-dimethylphenol and hexadecene at a molar ratio of 1.0 to 1.5:1. Put the amount into the reactor, add activated carbon supported sulfuric acid catalyst, the amount of catalyst is 5 to 20% of the mass of hexadecene, stir and heat up to 100 to 160°C under nitrogen protection, the reaction time is 8 to 30 hours, and recover the solid catalyst after filtration. The filtrate was evaporated under reduced pressure to remove unreacted raw materials, and then 2,4-dimethyl-6-(1-methyl-pentadecyl)phenol was evaporated under reduced pressure at 180-200°C under 1-2 mmHg. The product yield reaches more than 88%, and the content reaches more than 96%.
3. Used in the synthesis of vortioxetine
Vortioxetine is a new antidepressant drug developed by Lundbeck Pharmaceuticals of Denmark and Takeda Pharmaceuticals of Japan. It was approved by the U.S. Food and Drug Administration (FDA) in September 2013 for the treatment of major depression in adults. treat.
Step 1, synthesize 2-(2,4-dimethylphenylsulfanyl)chlorobenzene, specifically:
Prepare the reaction flask, vent nitrogen into the flask, add 2-chlorothiophenol (formula I) and 2,4-dimethylphenol into the reaction flask at a molar ratio of 1:1, add A certain amount of ethyl acetate, nickel nanopowder, sodium isopropoxide and anhydrous sodium sulfate; stir at room temperature for 10 to 30 minutes, raise the temperature to 40 to 60°C, stir and react for 6 to 10 hours, monitor the reaction progress with TLC, and wait until the reaction is completed , stop heating, wait until the reaction solution reaches room temperature, then filter, wash the filtrate 2 to 4 times with water, take the organic phase, add anhydrous sodium sulfate to dry overnight, filter, use a rotary evaporator to vacuum the filtrate with a vacuum of 0.06 to 1MPa, 40 to 60 The solvent is evaporated at ℃ to obtain 2-(2,4-dimethylphenylsulfanyl)chlorobenzene;
Step 2, prepare vortioxetine, specifically:
Take the reaction flask, add nitrogen, and add bis(dibenzylideneacetone)palladium and 1,1′-binaphthyl-2,2′ in a molar ratio of 1:2:390:1400~1420. – Add bisdiphenylphosphine, sodium tert-butoxide and toluene into the reaction bottle, mix and stir for 0.5 to 1.5 hours, add 2-(2,4-dimethylphenylsulfanyl)chlorobenzene prepared in step 1, and Add ethylenediamine at a molar ratio of 3:1 to 2-(2,4-dimethylphenylsulfanyl)chlorobenzene and heat to reflux, react for 2 to 4 hours, stop heating, cool to room temperature, and add diatoms Stir the soil for 15 to 25 minutes, filter, transfer the filtrate to the reaction bottle, pass hydrogen into the bottle, and add it at a molar ratio of 1:10 to 2-(2,4-dimethylphenylsulfanyl) chlorobenzene. For nickel nanopowder, heat up to 40-60°C and stir for 8-12 hours. Monitor the reaction progress with TLC. After the reaction, stop heating, lower to room temperature, filter, wash the reaction solution with water 2-4 times, and then use 30% sodium chloride. Wash the aqueous solution 1 to 3 times, take the organic phase, add anhydrous sodium sulfate, dry, and filter. The filtrate is evaporated with a rotary evaporator at a vacuum of 0.06 to 1 MPa and 40 to 60°C to remove the solvent to obtain vortioxetine.
Main reference materials
[1] CN201810102442.5 A separation process for extracting 2,4-dimethylphenol and 2,5-dimethylphenol from crude phenol
[2] CN201310347375.0 Asymmetric hindered phenol antioxidant and its synthesis method
[3] Preparation method of CN201410813720.X 2,4-dimethyl-6-(1-methyl-pentadecyl)phenol
[4] CN201410729639.3 New method for preparing vortioxetine hydrobromide beta crystal form

 微信扫一扫打赏
微信扫一扫打赏

