Background and overview[1][2]
Sodium p-fluorobenzene sulfinate is an important pharmaceutical intermediate, and its purity plays a vital role in the production of subsequent drugs. However, the current production technology of sodium p-fluorobenzene sulfinate still has process limitations. It has shortcomings such as complexity, low purity, and low yield. A sodium p-fluorobenzene sulfinate production process that can increase the yield and purity of sodium p-fluorobenzene sulfinate has not yet been discovered and remains to be developed.
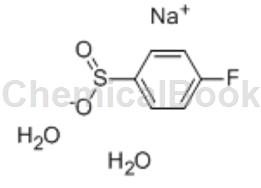
Sodium p-fluorobenzene sulfinate
Apply[3]
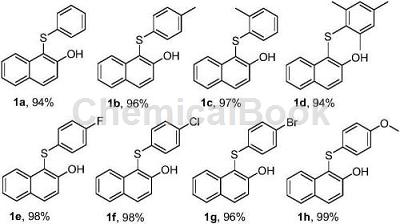
1. Preparation of aromatic thioether compounds
Take 0.5mmol 2-naphthol (72.1mg), 0.75mmol sodium benzene sulfinate (123.1mg), sodium p-toluenesulfinate (133.6mg), sodium o-toluenesulfinate (133.6mg), 2 , Sodium 4,6-trimethylbenzenesulfinate (154.7mg), Sodium p-fluorobenzenesulfinate (136.6mg), Sodium p-chlorobenzenesulfinate (149.0mg), Sodium p-bromobenzenesulfinate (180.8mg ) or sodium p-methoxybenzene sulfinate (145.6mg), 1.2mmol hydrochloric acid (36.5% hydrochloric acid 102.6μL) in a pressure-resistant tube, add 0.5mL ionic liquid, react at 80°C for 6h, react After completion, add 2 mL of ethyl acetate and extract three times, then rotary evaporate to remove the organic solvent and pass through the column layer of silica gel to obtain the target product 1a-1h. The yields are as follows: 94%, 96%, 97%, 94%, 98 respectively. %, 98%, 96%, 99%.
2.Synthesis of (E)-1-((2-chloro-2-styryl)sulfonyl)-4-methylbenzene
Add 0.20mmol phenylacetylene, 0.30mmol sodium p-fluorobenzene sulfinate, 0.40mmol ferric trichloride hexahydrate, and 2.0mL trifluoroethanol solvent into the reactor. Under a nitrogen atmosphere, heat to 80°C, continue stirring for 3 hours, stop the reaction, cool to room temperature, extract with dichloromethane, dry, and distill the solvent under reduced pressure. The crude product is separated by column chromatography to obtain the target product, with a yield of 94%. . 1HNMR (400MHz, CDCl3): δ7.43(d,J=7.9Hz,2H),7.35(d,J=7.1Hz,1H),7.32-7.30(m,3H),7.28(s,1H),7.13 (d,J=7.8Hz,2H),6.85(s,1H),2.32(s,3H).
Preparation[2]
Step 1. Synthesis of p-fluorobenzenesulfonyl chloride:
(1) Synthesis:
Into the enamel reaction kettle, add a certain amount of chlorosulfonic acid to lower the temperature of the materials in the kettle to 10-20°C. Start adding fluorobenzene dropwise, and use the dripping speed to control the reaction temperature below 25°C. After the reaction is completed, Stir at this temperature for 0.5 hours, and then suck the generated p-fluorobenzenesulfonyl chloride feed liquid into the high-level tank for later use. The molar ratio of fluorobenzene to chlorosulfonic acid is 1:4;
(2) Hydrolysis:
Add a certain amount of tap water to the enamel reaction kettle to cool down, then slowly add p-fluorobenzenesulfonyl chloride generated in step (1) dropwise, and use the dropping speed to control the reaction temperature below 30°C. After the dropwise addition is completed, continue stirring 5 minutes;
(3) Centrifugal separation:
Slowly put the hydrolyzed p-fluorobenzenesulfonyl chloride into the centrifuge, spin dry, bag, and submit for inspection for later use;
Step 2, synthesis and neutralization reaction of sodium p-fluorobenzene sulfinate:
(1) First put a certain amount of water into the kettle, then add anhydrous sodium sulfite to fully dissolve it, continue heating to raise the temperature of the material to 80°C, close the steam valve to stop heating, and start adding p-fluorobenzenesulfonyl chloride and Liquid caustic soda, PH value is 7.0-8.0, control the reaction at 78-80°C, the reaction reaches the end point, continue to keep the temperature at 80-85°C and stir for 30 minutes, the maximum reaction temperature does not exceed 85°C, sodium p-fluorobenzene sulfinate and no The molar ratio of water to sodium sulfite is 1:1;
(2) After the reaction in the previous step is completed, open the circulating water inlet and outlet valve of the reactor to reduce the material to 35±2°C. Use hydrochloric acid to adjust the pH value to 1.0. After adding the hydrochloric acid, stir for 30 minutes;
(3) Slowly put the hydrolyzed p-fluorobenzene sulfinic acid into a centrifuge, spin dry and bag;
(4) Add a certain amount of water to the enamel reactor, add the dried p-fluorobenzene sulfinic acid, and start stirring to cool the material to 20°C. Start dripping liquid alkali to adjust the pH value of the material to 7.0. -8.0, after adding alkali, control the temperature to 20°C. After stirring for 20 minutes, if the pH value does not change, the reaction is complete;
Step 3, post-processing process
(1) Put the reacted material into the concentration tank, turn on the vacuum system, open the steam valve to concentrate under reduced pressure. When the Baume degree of the material is 23-25°Be, stop heating, and let the concentration tank evacuate to normal pressure. The material remains still and settles for 10 minutes;
(2) Pump the material into the crystallization tank, and at the same time turn on the stirring of the crystallization tank, and open the circulating water inlet and outlet door to cool down. When the crystallization temperature reaches 30°C, start to shake off the material, dry the crystals, and submit for inspection.
Main reference materials
[1] Wei Yang, Huang Xi, Liu Jingjing. (2013). Simultaneous determination of sodium p-toluenesulfinate and sodium p-toluenesulfinate by high performance liquid chromatography. Pesticide Science and Management, 34(12), 48- 52.
[2] Wang Xu, Zhao Heli, Wang Jiajun. (2015). A production process of sodium p-fluorobenzene sulfinate.
[3] Zhao Yan. (1999). “One-pot” synthesis of tosmic from sodium p-toluenesulfinate. Chemical Engineer (1), 15-16.

 微信扫一扫打赏
微信扫一扫打赏

