Background and overview[1]
4-Methylphenylglyoxylic acid can be used as a pharmaceutical synthesis intermediate. If 4-methylphenylglyoxylic acid is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if the eye contact If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
Ir/SpiroPAP catalyzes the direct asymmetric acid hydrogenation of αketo to generate chiral α-hydroxy acids including 4-methylphenylglyoxylic acid:
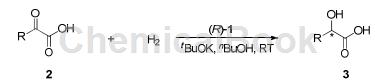
(R=3-methylphenyl-)
Benzoylformic acid (2a) was used as a model substrate to optimize reaction conditions. The effect of base amount, solvent and ligand on reactivity and reactivity is screened for enantioselectivity. The reaction rate can be accelerated by adding 1.06 equivalents of tBuOK, and complete conversion is obtained within 2 to 75 hours. Solvent screening showed that n-butanol gave the best enantioselectivity. By comparing various chiral SpiroPAP ligands, it was found that the introduction of an alkyl ligand’s pyridine ring at the 6-position reduces the enantioselectivity. However, the presence of an alkyl group at the 3- or 4-position of the pyridine ring can improve the enantioselectivity. Selectivity. Increasing the substrate concentration from 0.4 M to 1.0 M resulted in slightly lower enantioselectivity. Based on the above results, the reaction was optimized. Therefore, the conditions were set as follows: 0.1 mol% of (R)-1c catalyst, 1.06 equivalents of tBuOK as base, nBuOH as solvent (substrate concentration 0.4 M at room temperature). For the more sterically hindered omethylbenzoylformic acid (2c), the hydrogenation was extremely rapid and highly enantioselective. Complete conversion was completed within 1 hour to give 4-methylphenylglyoxylic acid 98% ee.
Main Reference
[1] Yan P C, Xie J H, Zhang X D, et al. Direct asymmetric hydrogenation of α-keto acids by using the highly efficient chiral spiro iridium catalysts[J]. Chemical Communications, 2014, 50(100): 15987 -15990.

 微信扫一扫打赏
微信扫一扫打赏

