Overview[1]
O-bromotoluene is a colorless liquid at room temperature. Insoluble in water, soluble in ethanol, ether and benzene, miscible in carbon tetrachloride. After oxidation, o-benzoic acid can be generated. Its preparation uses o-toluidine as raw material and is obtained by diazotization and replacement. It can be used as raw materials for organic synthesis, pharmaceutical intermediates, etc. Osobenzene has a certain degree of toxicity and can be poisoned by inhalation, ingestion or transdermal absorption. It is highly irritating to the eyes and skin. Oral administration, skin absorption and respiratory inhalation of toluene vapor can cause acute poisoning. In addition, otoluene has a strong irritating and corrosive effect on the eyes and skin, and is destructive to the environment.
Multiphoton ionization mass spectrometry[1]
In the experiment, lasers with wavelengths of 335nm and 266nm were used to perform multiphoton ionization of orthotoluene, and the resulting mass spectra are shown in Figures 3.1 and 3.2. According to the chemical structural formula of ortho-olfactory toluene, by analyzing each mass spectrum peak in the MPT-MS chart produced under the action of 335nm laser, we believe that under the action of 355nm laser, due to the non-idealization of the experimental instrument, the fragment ions generated are mainly It comes from the ionization and dissociation of impurity molecules, while there is no obvious ionization and dissociation of o-toluene molecules. Therefore, we conducted the following experiments using laser.
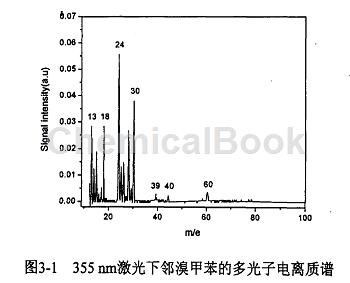
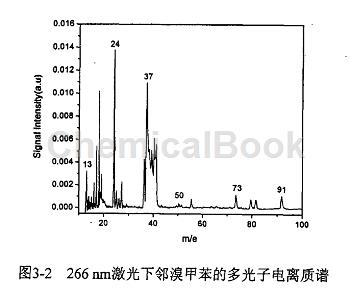
The experiment measured the multi-photon ionization time-of-flight mass spectrum of o-bromotoluene when the laser wavelength was 266 nm, as shown in Figure 1. The pressures of the Fort East source chamber and the ionization chamber after the experiment were 6.8X10-* Pa and 4.4X10~ respectively. * Pa. In the TOF mass spectrum of o-bromotoluene, the main mass spectrum peaks are CH+ (13), CH2 (14), CH (15); CI (24), C2H+ (25), C2H2 (26), Cf ( 36), CH+(37), CH2 (38), CH (39):C.H+ (49), CH(50), C.H (51), C.Hj (63), C;H (65), C:H+(73) .C,H/ (91), Br+(79) and Br+ (81) are clearly distinguished from the mass spectrum. Their intensity ratio is about 1↑1. No o-bromotoluene was found in the experiment. Cluster ions and parent molecular ions. As can be seen from the figure, C (24) ion signal intensity is the strongest, followed by CjH+ (37) and other ion signal intensity is weak. We measured the mass spectra at different laser intensities. From the experimental results It can be seen that the types of fragment ions produced do not change with the change of laser intensity, but the relative intensity of each ion signal changes.
Conclusion[1]
This paper uses a time-of-flight mass spectrometer under the action of 266 nm laser to study the multiphoton ionization and dissociation mass spectrometry of o-bromotoluene, and discusses its multiphoton ionization and dissociation mechanism. The results show that the The multiphoton process belongs to type B mechanism. The o-bromotoluene molecule may absorb a photon, directly open the C-Br bond, and dissociate the Br atom. At the same time, the o-bromotoluene molecule may first cross the barrier to isomerize, and then dissociate. Remove the Br atom. Therefore, the CH7 ion peak seen in the mass spectrum may have three isomers (tolyl ions, benzyl ions, and chromium ions). Fragment ions such as C:H+, CH, and CH are composed of The C and Ht ions obtained through the above two channels are further ionized and dissociated by absorbing one or more photons.
References
[1] Zhu Guiwen. Research on the gas-phase ammoxidation of o-bromotoluene to o-bromobenzonitrile[D]. Wuhan University of Technology, 2007.
[2]Song Bao, Kong Xianghe, Liu Yumei, Su Guangyong, Lang Jigang, Zhang Shudong. Multiphoton ionization mass spectrometry of o-bromotoluene and ab initio calculations[J]. Acta Atomic and Molecular Physics, 2009, 26(06):1021- 1026.

 微信扫一扫打赏
微信扫一扫打赏

